The Internet Journal of Thoracic
and Cardiovascular Surgery TM ISSN: 1524-0274
P. J Overwalder, M.D.
Department of Surgery I
Division of Cardiac Surgery
University Hospital Graz
Citation:
P. J Overwalder: Intra Aortic Balloon
Pump (IABP) Counterpulsation . The Internet Journal of Thoracic
and Cardiovascular Surgery. 1999. Volume 2 Number 2.
Table of Contents
History
Physiologic Effects of IABP Therapy
Control of the IABP
Insertion Techniques
Complications
Experience at a Single Center
References
History
In 1958 Harken described for the first time
a method to treat left ventricular failure by using counterpulsation
or diastolic augmentation. He suggested removing a certain blood
volume from the femoral artery during systole and replacing
this volume rapidly during diastole. By increasing coronary
perfusion pressure this concept would therefore augment cardiac
output and unload the functioning heart simultaneously 1 , 2
. This method of treatment was limited because of problems with
access (need for arteriotomies of both femoral arteries), turbulence
and development of massive hemolysis by the pumping apparatus.
Even experimental data showed that no augmentation of coronary
blood flow was obtained 3 .
Then in the early 1960s Moulopoulus et al.
4 , 5 from the Cleveland Clinic developed an experimental prototype
of the intra-aortic balloon (IAB) whose inflation and deflation
were timed to the cardiac cycle. In 1968 the initial use in
clinical practice of the IABP and it`s further improvement was
realized resp. continued by A. Kantrowiz`s group 6 , 7 .
In its first years, the IABP required surgical
insertion and surgical removal with a balloons size of 15 French.
In 1979 after subsequent development in IABP technology a dramatic
headway with the introduction of a percutaneous IAB with a size
of 8,5 to 9,5 French was achieved 8 , 9. This advance made it
for even nonsurgical personnel possible, to perform an IAB insertion
at the patientís bedside. In 1985 the first prefolded IAB was
developed.
Today continued improvements in IABP technology
permit safer use and earlier intervention to provide hemodynamic
support. All these progresses have made the IABP a mainstay
in the management of ischemic and dysfunctional myocardium.
Physiologic Effects of IABP Therapy
After correct placement of the IAB in the
descending aorta with it`s tip at the distal aortic arch (below
the origin of the left subclavian artery) the balloon is connected
to a drive console. The console itself consists of a pressurized
gas reservoir, a monitor for ECG and pressure wave recording,
adjustments for inflation/deflation timing, triggering selection
switches and battery back-up power sources. The gases used for
inflation are either helium or carbon dioxide . The advantage
of helium is its lower density and therefore a better rapid
diffusion coefficient. Whereas carbon dioxide has an increased
solubility in blood and thereby reduces the potential consequences
of gas embolization following a balloon rupture.
Inflation and deflation are synchronized to
the patientsí cardiac cycle. Inflation at the onset of diastole
results in proximal and distal displacement of blood volume
in the aorta. Deflation occurs just prior to the onset of systole
(Fig. 152-a) .
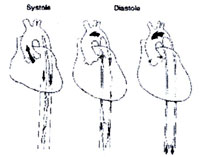
Figure 152-a
click to enlarge
Intra aortic balloon (IAB) during
systole and diastole
The primary goals of IABP treatment are to
increase myocardial oxygen supply and decrease myocardial oxygen
demand. Secondary, improvement of cardiac output (CO), ejection
fraction (EF), an increase of coronary perfusion pressure, systemic
perfusion and a decrease of heart rate, pulmonary capillary
wedge pressure and systemic vascular resistance occur 10 , 11
, 12 (Tab.1 goals of IABP)
There are several determinants of oxygen supply
and demand (Tab.2 Determinants of O2 Supply and Demand).
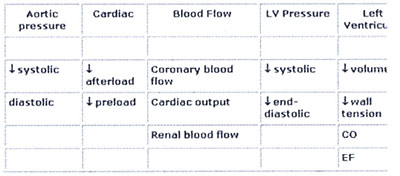
Table 1: Hemodynamic effects of IABP Therapy
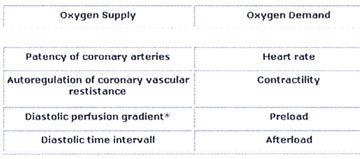
Table 2: Determinants of Myocardial Oxygen Supply
and Demand
In particular systolic wall tension uses approximately
30% of myocardial oxygen demand. Wall tension itself is affected
by intraventricular pressure, afterload, end-diastolic volume
and myocardial wall thickness. Regarding to the studies of Sarnoff
et al. the area under the left ventricular pressure curve, TTI
(= tension-time index ), is an important determinant of myocardial
oxygen consumption 13. On the other hand, the integrated pressure
difference between the aorta and left ventricle during diastole
(DPTI = diastolic pressure time index) represents the myocardial
oxygen supply (i.e. hemodynamic correlate of coronary blood
flow) 14 , 15 .
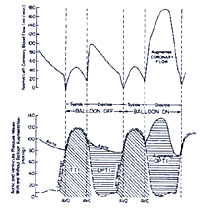
Figure152-b
click
to enlarge
Schematic representation of
coronary blood flow, aortic and left ventricular pressure wave
form with / without IABP. (Effects on DPTI and TTI . Balloon
inflation during diastole augments diastolic pressure and increases
coronary perfusion pressure as well as improving the relationship
between myocardial oxygen supply and demand (DPTI:TTI ratio)
Control of the IABP
TRIGGERING
To achieve optimal effect of counterpulsation,
inflation and deflation need to be correctly timed to the patientís
cardiac cycle. This is accomplished by either using the patientís
ECG signal, the patientís arterial waveform or an intrinsic
pump rate. The most common method of triggering the IAB is from
the R wave of the patientís ECG signal. Mainly balloon inflation
is set automatically to start in the middle of the T wave and
to deflate prior to the ending QRS complex. Tachyarrhythmias,
cardiac pacemaker function and poor ECG signals may cause difficulties
in obtaining synchronization when the ECG mode is used. In such
cases the arterial waveform for triggering may be used.
TIMING and WEANING
It is important that the inflation of the
IAB occurs at the beginning of diastole, noted on the dicrotic
notch on the arterial waveform. Deflation of the balloon should
occur immediately prior to the arterial upstroke. Balloon synchronization
starts usually at a beat ratio of 1:2. This ratio facilitates
comparison between the patientís own ventricular beats and augmented
beats to determine ideal IABP timing. Errors in timing of the
IABP may result in different waveform characteristics and a
various number of physiologic effects (Fig. 152-c).
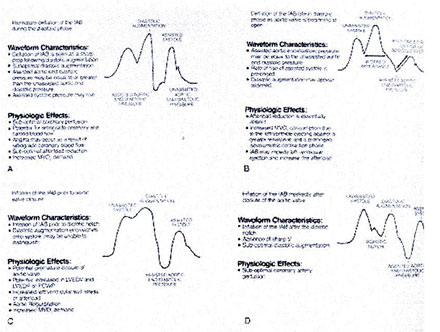
Figure 152-c: Arterial pressure wave form alterations
associated with inflation and deflation of the IAB
If the patientís cardiac performance improves,
weaning from the IABP may begin by gradually decreasing the
balloon augmentation ratio (from 1:1 to 1:2 to 1:4 to 1:8) under
control of hemodynamic stability . After appropriate observation
at 1:8 counterpulsation the balloon pump is removed.
Indications and Contraindications
Early purposed indications for intraaortic
balloon pumping have included cardiogenic shock or left ventricular
failure, unstable angina, failure to separate a patient from
cardiopulmonary bypass and prophylactic applications, including
stabilization of preoperative cardiac patients as well as stabilization
of preoperative noncardiac surgical patients 10, 17 , 18 , 19
, 20 , 21 . Today more extending indications are: Cardiac patients
requiring procedural support during coronary angiography and
PTCA, or as a bridge to heart transplantation. Further on in
pediatric cardiac patients and as well as in patients with stunned
myocardium, myocardial contusion, septic shock and drug induced
cardiovascular failure the IABP can be life-saving 22 , 23 ,
24 , 25 , 26 , 27 , 28 , 29 , 30 , 31
IABP therapy should only be considered only
for use in patients who have the potential for left ventricular
recovery, or to support patients who are awaiting cardiac transplantation.
Absolute contraindications of IABP are relatively few (Tab.3
Contraindications of IABP). There are successful reports of
its usage in patients with aortic insufficiency 32 , 33 and
in patients with acute trauma to the descending aorta 34 .
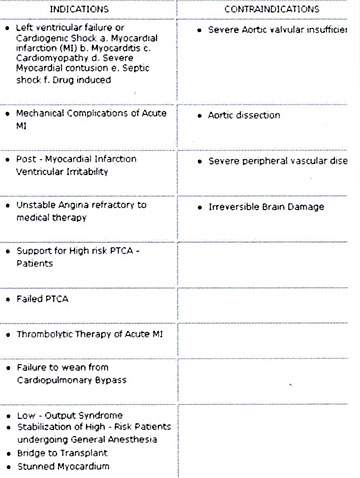
Table 3: IABP Counterpulsation Indications and
Contraindications
Insertion Techniques
In the early years of IABP - therapy, insertion
of the balloon was performed by surgical cut down to the femoral
vessels. After a longitudinal incision in the groin, the femoral
arteries were identified and controlled. A vascular graft was
then sewn to the common femoral artery in an end-to-side fashion.
The balloon was introduced into the artery via the graft and
properly positioned in the thoracic aorta and the graft tightly
secured to the distal portion of the balloon catheter. Finally
the skin incision was closed. Removal of the balloon required
a second operation.
Since 1979, a percutaneous placement of the
IAB via the femoral artery using a modified Seldinger technique
allows an easy and rapid insertion in the majority of situations.
After puncture of the femoral artery a J-shaped guide wire is
inserted to the level of the aortic arch and then the needle
is removed. The arterial puncture side is enlarged with the
successive placement of an 8 to 10,5Fr dilator/sheath combination.
Only the dilator needs to be removed.
Continuing, the balloon is threaded over the
guide wire into the descending aorta just below the left subclavian
artery. The sheath is gently pulled back to connect with the
leak-proof cuff on the balloon hub, ideally so that the entire
sheath is out of the arterial lumen to minimize risk of ischemic
complications to the distal extremity. Recently sheathless insertion
kits are available. Removal of a percutaneously placed IAB may
either be via surgical removal or closed technique. There are
alternative routes for balloon insertion. In patients with extremely
severe peripheral vascular disease or in pediatric patients
the ascending aorta or the aortic arch may be entered for balloon
insertion 35 , 36 . Other routes of access include subclavian,
axillary or iliac arteries 37 , 38 , 39 .
Complications
Although the incidence of complications has
decreased significantly as experience with the device has increased,
IABP therapy in todayís patients` population does still hold
a risk for complications (Tab. 4). Because todayís patient population
is elderly (68 - 80 years), very often female and may suffer
from severe peripheral vascular disease and hypertension or
diabetes. The most common vascular complication is limb ischemia.
It may occur in 14-45% of patients receiving IABP therapy 40
, 41. Therefore the patient must be consistently observed for
any symptoms of ischemia during IABP counterpulsation. If signs
of ischemia appear the balloon should be removed. In general,
vascular injuries should be dealt with directly by surgical
interventions and repair. Balloon related problems and infection
require removal and / or replacement of the IAB .
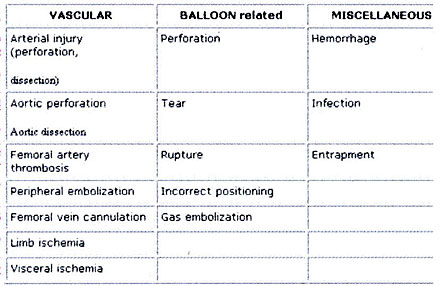
Table 4: Complications of IABP counterpulsation
Experience at a Single Center
Treatment of low cardiac output syndrome using
IABP counterpulsation has been used at our institution since
1983. Till December 1993 a total number of 440 patients (pts)
(9,95%) out of 4420 patients, who underwent cardiac surgery
procedures with the use of cardiopulmonary bypass, were supported
with an IABP. (Age distribution : Tab. 6) There were 294 male
and 146 female patients. Overall survival rate after implantation
of the IABP was 75% (n=330 pts) .
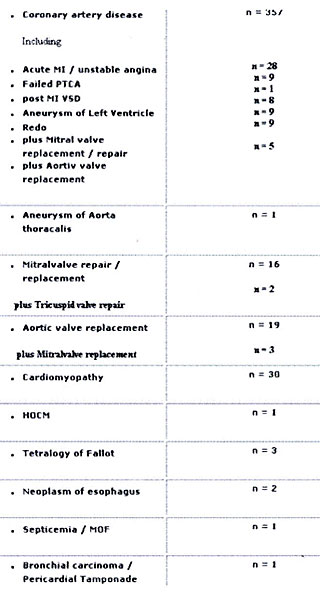
Table 5: Diagnosis prior to IABP implantation
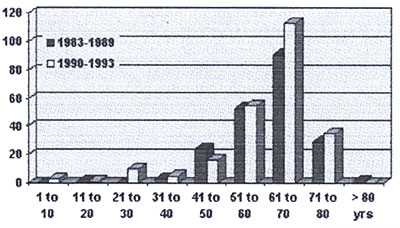
Table 6: Age Distribution of IABP patients
In the early years (1983-1989) as method of
choice, implantation of the balloon was performed via a surgical
cut down of the femoral artery. Complications were observed
in 20 pts (8.4%) : In 9 pts (3.7%) positioning of the balloon
was impossible due to severe vascular disease, 5 pts (2.1%)
developed a thrombosis of the femoral artery and 1 patient (0.4%)
died because of untreatable thrombosis of the mesenteric artery.
Hospital mortality in this group was 36% (survival rate of 64%).
Mean pumping time was 3 days (1 - 15).
Since 1990 we prefer the percutaneous insertion
of the device. After a learning curve more than 90% of 202 patients
received an IABP using this technique. Complication rate was
less than 8% (mainly leg ischemia with amputation of the leg
in 1 patient, 3 infections of the puncture point and 4 cases
of impossible positioning of the balloon ). Survival rate was
68.5% (hospital mortality of 31.5%) . 278 pts (63%) received
the balloon pump at the operating theater - mainly because of
failure to wean from cardiopulmonary bypass -151 pts (34,3%)
at an intensive care unit and 11 pts (2,5%) as a bridge to transplant.
Table 6 shows a detailed list of all various diagnoses prior
to IABP therapy .
References
1. Harken DE (1958) Presentation at the International
College of Cardiology, Brussels, Belgium
2. Harken DE (1976) Circulatory assist devices.
Med Instrum 10: 215
3. Dormandy JA, Goetz RH, Kripke DC (1969)
Hemodynamics and coronary blood flow with counterpulsation.
Surgery 65: 311
4. Moulopoulos SD, Stephen R, Topaz S et al
(1962) Extracorporeal assistance to the circulation and intraaortic
ballon pumping. Trans Am Soc Artif Int Org 7: 85
5. Moulopoulos SD, Topaz S, Kolff WJ (1962)
Diastolic balloon pumping (with carbon dioxide) in the aorta
- a mechanical assistance to the failing circulation. Am Heart
J 63: 669
6. Kahn JK, Rutherford BD, McConahay DR (1990)
Supported High Risk coronary angioplasty using intraaortic balloon
pump counterpulsation. J Am Coll Cardiol 15: 1151
7. Kantrowitz A, Tjonneland S, Freed PS et
al (1968) Inital clinical experience with intra-aorta balloon
pumping in cardiogenic shock. JAMA 203: 113
8. Bregman D, Casarella WJ, (1981) Percutaneous
intraaortic balloon pumping: Initial clinical experiences. Ann
Thorac Surg 29: 153
9. Hauser AM, Gordon S, Ganzadharen V et al
(1982) Percutaneous intraaortic balloon counterpulsation . Clinical
effectiveness and hazards. Chest 82: 422
10. Bolooki H (1984) Clinical application of
Intra-Aortic Ballon Pump. Mount Kisco, NY, Futura Publishing
11. Sarnoff SJ, Braunwald E, Welch GH et al
(1958) Hemodynamic determinants of oxygen consumption of the
heart with special reference to the tension time index. Am J
Physiol 192: 148
12. Akyurekli Y, Taichmann JC, Keon WJ (1980)
Effectivness of intra aortic balloon counteroulsation and systolic
unloading. Can J Surg 23: 122
13. Pennington DG, Swartz MT (1990 ) Mechanical
circulatory support prior to cardiac transplantation. Sem Thor
& Cardiovasc Surg 2(2): 125
14. Grotz RL, Yeston NS (1989) Intraaortic
balloon counterpulsation in high risk cardiac patients undergoing
non cardiac surgery. Surgery 106: 1
15. Hoffman JIE, (1978) Determinants of prediction
of transmural myocardial perfusion Circulation 58: 381
16. Lembo NJ (1989) Failed angioplasty and
intraaortic balloon pumping. Cardiac assist 5(1): 5
17. Ayers Sm, (1988) The prevention and trearment
of shock in acute myocardial infarction. Chest 93 (suppl): 17S
18. Bolooki H (1989) Emergency cardiac procedures
in patients in cardiogenic shock due to complications of coronary
artery disease. Circulation 79 (suppl I): I-137
19. Georgen RF, Diertrick JA, Pifarre R, et
al. (1989) Placement of intraaortic balloon pump allowing definitive
surgery on patients with severe cardiac disease. Surgery 106:
808
20. Golding LAR, Loop FD, Petes M, et al. (1980)
Late survival following use of intra-aortic balloonpumping in
revascularization surgery. Ann Thorac Surg 30: 48
21. Grotz RL, Yeston NS (1989) Intraaortic
balloon counterpulsation in high risk cardiac patients undergoing
non cardiac surgery. Surgery 106: 1
22. Anwar A, Mooney MR, Sterzer SH (1990 )
Intra-aortic balloon counterpulsation support for elective coronary
angioplasty in the setting of poor left ventricular function:
A two center experience. J.Invas.Cardiol.1(4): 175
23. Bavaria JE, Furukawa S, Kreiner G (1990)
Effect of circulatory assist devices on stunned myocardium.
Ann Thorac Surg 49: 123
24. Freedberg RS, Friedmann GR, Palu RN, et
al. (1987) Cardiogenic shock due to anti-histamine overdose.
Reversal with intraaortic balloon counterpulsation JAMA 257:
660
25. Iberer F, Roupec R, Dacar D, et al (1990)
Surgical Implantation of the intra-aortic balloon pump via the
arteria femoralis . Angio 12(2) : 43
26. Lamberti JJ, Cohn LH, CollinsJJ Jr, (1974)
Iliac artery cannulation for intraaortic balloon counterpulsation.
J Thorac Cardiovasc Surg 67: 976
27. Lane AS, Woodward AC, Goldman MR (1987)
Massive propranolol overdose poorly responsive to pharmacologic
therapy: Use of the intra aortic balloon pump. Ann Emerg Med
16(12): 1381
28. McBride LR, Miller LW, Nauheimer KS, et
al. (1989) Axillary artery insertion of an intraaortic balloon
pump. Ann Thorac Surg 48: 874
29. Mercer D, Doris P, Salerno TA (1981) Intra-aortic
counterpulsation in septic shock Can J Surg 24(6): 643
30. Ohmann EM, Califf RM, George BS, et al.
(1991) The use of intraaortic balloon pumping as an adjunct
to reperfusion therapy in acute myocardial infarction Am Heart
J 121: 895
31. Shirkey AL, Loughridge BP, Lain KC (1976)
Insertion of the intraaortic balloon through the aortic arch.
Ann Thorac Surg 21: 560
32. Vigneswaran WT, Reece IJ, Davidson KG (1985)
Intraaortic balloon pumping : seven years`experience. Thorax
40: 858
33. Yellin E, Levy I, Bregman D, et al (1973)
Hemodynamic effects of intraaortic balloon pumping in dogs with
aortic incompetence. Trans Am Soc Artif Intern Org 19: 389
34. Ammons MA, Moore EE, Moore FA, et al (1990)
Intraaortic balloon pump for combined myocardial contusion and
thoracic aortic rupture. J Trauma 30: 1606
35. Gueldner TL, Laurence GH (1975) Intraaortic
balloon pumping in children. Ann Thorac Surg 19: 88
36. Shaw J, Taylor DR, Pitt B (1974) Effects
of intraaortic balloon counterpulsation in regional coronary
blood flow. Am J Card 34: 552
37. KantrowitzA, Wasfie T, Freed PS, et al.
(1986) Intraaortic balloon pumping 1967 through 1982: Analysis
of complications in 733 patients. Am J Cardiol 57: 976
38. Maccioli GA, Lucas WJ, Norfleet EA (1988)
The intra-aortic balloon pump: A review. J. Cardiothor. Anesth.
2: 365
39. Mayer JH (1978) Subclavian artery approach
for insertion of intra-aortic Balloon J Thorac Cardiovasc Surg
76: 61
40. Kantrowitz A, Tjonneland S, Krakauer J
et al (1968) Clinical experience with cardiac assistance by
means of intra aortic phaseshift balloon pumping. Trans Am Soc
Artif Intern Org 14: 344
41. Mayer JH (1978) Subclavian artery approach
for insertion of intraaortic Balloon J Thorac Cardiovasc Surg
76: 61
RIGHT VENTRICULAR HEART
FALURE
OBSTRUCTIVE
SLEEP APNEA, HYPOVENTILATION, PULMONARY HYPERTENSION, RIGHT
VENTRICULAR FAILURE
There are numerous respiratory
complications of obesity, including an increased breathing workload,
respiratory muscle inefficiency, decreased functional reserve
capacity and expiratory reserve volume, and closure of peripheral
lung units.These complications often result in a ventilation-perfusion
mismatch, especially in the supine position. Obesity is a classic
cause of alveolar hypoventilation. Historically, the obesity-hypoventilation
syndrome has been described as the "pickwickian" syndrome,
and obstructive apnea was first observed in patients with severe
obesity. Sleep apnea is defined as repeated episodes of obstructive
apnea and hypopnea during sleep, together with daytime somnolence
and/or altered cardiopulmonary function. The prevalence of sleep-disordered
breathing and sleep disturbances rises dramatically in obese
subjects and obesity is by far the most important modifiable
risk factor in sleep-disordered breathing. It has been estimated
that 40 million Americans suffer from sleep disorders and that
the vast majority of these patients remain undiagnosed.
Despite careful screening by history
and physical examination, sleep apnea is revealed only by polysomnography
in most patients. Although, some clinical features could be
useful in screening for sleep apnea, the diagnostic accuracy
is inadequate.
Patients with sleep apnea have an increased risk of diurnal
hypertension, nocturnal dysrhythmias, pulmonary hypertension,
right and left ventricular failure, myocardial infarction, stroke,
and mortality. The prevalence of pulmonary hypertension in subjects
with obstructive sleep apnea is 15 to 20 percent; however, pulmonary
hypertension rarely is observed in the absence of daytime hypoxemia.
According to Kessler and colleagues the extent of pulmonary
hypertension in patients with obstructive sleep apnea is generally
mild to moderate (pulmonary artery pressures ranging between
20 and 35 mmHg) and does not necessitate specific treatment.
Although there is a link between sleep apnea and systemic hypertension,
the association of obesity with both disorders confounds the
relationship. A physician who evaluates an obese patient who
has been referred for hypertension should address related symptoms
such as habitual snoring, nocturnal gasping or choking, witnessed
episodes of apnea, and daytime sleepiness. It is important to
remember, however, that the clinical and ECG signs of cor pulmonale
appear later than do those of pulmonary hypertension (assessed
by right heart catheterization). Numerous treatments are available
for sleep apnea, but weight loss in obese patients should always
be advocated.
Severe untreated sleep-disordered breathing
can further impair LV function, leading to arterial oxyhemoglobin
desaturation and arrhythmias.Central sleep apnea may occur in
as many as 40% of patients with heart failure, and 10% suffer
from obstructive sleep apnea. Obstructive sleep apnea increases
afterload and heart rate during sleep but is responsive to continuous
positive airway pressure.