Just like other muscles; the heart responds to exercise with increased efficiency. Occasionally the changes can be a bit unnerving.
The most important muscle an athlete develops is her heart. Generally as she becomes more fit, her resting heart rate slows - a sign that her heart is pumping blood with greater efficiency. In the first six to 12 weeks of training, resting heart rate decreases by five to 10 percent.
The resting pulse of a trained heart is usually less than 70 beats per minute. Many athletes monitor their heart rates while exercising and while at rest (either immediately upon awakening or after 10 to 15 minutes of inactivity). But sometimes the results are unsettling.
For instance, say you are checking your resting rate after watching TV for 15 minutes, and you notice a skip between beats. You continue to monitor your pulse, now rhythmic and steady; you're not sure if you should dial 911. A while later, the skip occurs again. All thoughts of getting your resting heart rate below 70 are out the window; and you become more concerned with whether you'll survive the night or not.
Skipped heartbeats are usually premature heartbeats - one beat quickly follows another, and the resulting pause in the rhythm of your normal heartbeat is assumed to be a "skipped" beat. Occasional premature beats do occur in healthy people and usually don't indicate a problem unless they're accompanied by chest pain, light-headedness or other symptoms.
If you experience a premature beat more than once every 20 to 30 minutes, however; or if you have an irregular heart rate, palpitations or pauses in your heartbeat, it's extremely important to see your physician. She can determine whether you may have a benign condition called athlete's heart or a more serious problem.
The term "athlete's heart" describes a collection of changes that occur as you train. The two most common findings in trained athletes are bradycardia, or a slow pulse (less than 70 beats per minute), and phasic sinus arrhythmia, a pulse that speeds and slows with respiration.
How common is it to have a pulse that speeds up and slows down when you breathe?
Up to 69 percent of aerobically trained athletes demonstrate phasic sinus arrhythmia. This benign rhythm discrepancy becomes more common as you become more fit; it temporarily disappears when you increase your heart rate with exercise.
Physicians have noted several other changes that reflect the heart's normal adaptation to training. Besides lowering the resting pulse rate, training makes the pulse more forceful, producing a harmless murmur as blood flows through the heart and blood vessels.
The athlete's heart may also appear slightly enlarged on a chest X-ray and an electrocardiogram (EKG) may chart patterns that would not show on the EKG of an untrained heart. However, these do not indicate disease.
Aerobics vs. Anaerobic Training
Like other muscles, your heart responds in a healthy way to specific training. if your training is principally aerobic, your heart must handle a large volume of blood. Its internal chambers will enlarge slightly and its overall size will increase.
The stroke volume - the amount of blood ejected from the chambers with each beat-will also increase, as your pulse rate decreases. These adaptations allow your heart to pump blood with maximum efficiency.
On the other hand, weight-lifting or resistance training will cause your heart muscle to thicken without enlargement of its cavity. This adaptation enables it to generate the increased blood pressure necessary for anaerobic exercise but doesn't contribute to a more efficient stroke volume or a lower pulse rate. If you combine aerobic and resistance training, your heart will of course show the benefits of both types of exercise.
Whatever type of training you do, changes in your heart muscle occur gradually over the first four to six weeks of consistent training. Aerobically trained athletes will notice this adaptation through their lower resting heart rates.
Some researchers have noted increased resting heart rates with overtraining. If your resting heart rate suddenly increases from 60 to 70 beats per minute and you are working harder than usual, watch out! You may be overtraining and need to slow down. When you stop training completely your heart will return to your untrained heart rate within three to four weeks.
While there are many benefits to aerobic conditioning of the heart muscle, a trained heart does not make you immune from heart problems.
Aerobically trained people have lower rates of cardiovascular disease, such as hypertension and coronary artery disease, but a trained heart is not immune to heart problems. The tragic deaths of Olympic volleyball player Flo Hyman, collegiate basketball player Hank Gathers and other young athletes in their prime are a sobering reminder that no one is invincible.
Still, sudden death is very rare. In a 20-year study done at the Air Force Academy on recruit deaths during exercise, the incidence was 1.7 deaths for 500,000 hours of exercise. The researchers concluded that "the risk of exercise-related sudden death is no greater than deaths occurring by chance alone." Most cases of sudden death in athletes under the age of 35 are due to pre-existing structural congenital heart disease.
In people over the age of 35, exercise-related sudden death is often caused by underlying coronary artery disease. Adaptations the heart makes during normal training don't cause any of these problems. Most of us have healthy hearts, and appropriate training can reduce one's risk of some forms of heart disease.
Even with a healthy heart, exercising with a high fever maybe risky. You might place undue strain on the heart while fighting an infection, or you might have a coexisting inflammation of the heart. Several other factors might indicate that you are at risk for an exercise-related cardiac problem. If any of the risk factors listed below apply to you, contact your doctor and get an evaluation before beginning a program.
Some cardiac risk factors:
I. A history of fainting for no apparent reason, especially if the fainting occurred during or immediately after exercise.
2. Symptoms of an irregular heartbeat, palpitations, skipped beats or fluttering heartbeat either when resting or exercising.
3. A close blood relative who died suddenly before the age of 55, or a family history of early coronary heart disease, high cholesterol, Marfan's syndrome or enlarged heart.
4. A family or personal history of seizures.
5. You are a male over age 40 or a female over 50, the American College of Sports Medicine recommends that you have a medical evaluation before you begin an exercise program.
Aerobically trained people have lower rates of cardiovascular disease, such as hypertension and coronary artery disease, but a trained heart is not immune to heart problems. The tragic deaths of Olympic volleyball player Flo Hyman, collegiate basketball player Hank Gathers and other young athletes in their prime are a sobering reminder that no one is invincible.
Still, sudden death is very rare. In a 20-year study done at the Air Force Academy on recruit deaths during exercise, the incidence was 1.7 deaths for 500,000 hours of exercise. The researchers concluded that "the risk of exercise-related sudden death is no greater than deaths occurring by chance alone." Most cases of sudden death in athletes under the age of 35 are due to pre-existing structural congenital heart disease.
In people over the age of 35, exercise-related sudden death is often caused by underlying coronary artery disease. Adaptations the heart makes during normal training don't cause any of these problems. Most of us have healthy hearts, and appropriate training can reduce one's risk of some forms of heart disease.
Even with a healthy heart, exercising with a high fever maybe risky. You might place undue strain on the heart while fighting an infection, or you might have a coexisting inflammation of the heart. Several other factors might indicate that you are at risk for an exercise-related cardiac problem. If any of the risk factors listed below apply to you, contact your doctor and get an evaluation before beginning a program.
Some cardiac risk factors:
I. A history of fainting for no apparent reason, especially if the fainting occurred during or immediately after exercise.
2. Symptoms of an irregular heartbeat, palpitations, skipped beats or fluttering heartbeat either when resting or exercising.
3. A close blood relative who died suddenly before the age of 55, or a family history of early coronary heart disease, high cholesterol, Marfan's syndrome or enlarged heart.
4. A family or personal history of seizures.
5. You are a male over age 40 or a female over 50, the American College of Sports Medicine recommends that you have a medical evaluation before you begin an exercise program.
The pages listed under this information section contain summary relevant topics written by many different experts in the field for the Montgomery Heart Foundation for Cardiomyopathy. The information contained in these summaries was originally collected in 1997 to together a printed brochure for patients, families and health care professionals. Although these summaries are now 6 or 7 years ( they have been re-reviewed and we believe contain basic and general information that is still helpful to patients and families today.
While the entity of the "athlete's heart" has been recognized for c years, only in the last two decades has the application of echocardiography and other noninvasive imagine techniques permitted definition wi precision of the alterations in cardiac dimensions associated with conditioning. Echocardiography has demonstrated that long-term training leads to an increase in left ventricular (LV) mass due to i LV diastolic cavity dimension, wall thickness, or both. These changes of cardiac morphology are relatively mild in absolute terms, and the between athlete and non-athlete populations are statistically sign generally small. Furthermore, cardiac alterations associated with differ somewhat depending on the particular sporting discipline in which individual athlete participates. In particular, the changes in LV wall thickness, cavity dimensions, or both associated with long-term training may be more striking in certain sports such as distance r swimming, cycling and rowing/canoeing. It is in athletes training in sports that the differential diagnosis is more likely to be raised.
Differential Diagnosis
Dilated Cardiomyopathy
In an important minority of athletes, the increase in LV end-diastc dimension that occurs with training overlaps that which is characteristic of certain pathologic entities. While LV cavity dimension in athletes is in the range of 53 to 58 mm, in some individuals it may extend in regarded as the pathological range of >60 mm (up to 70 mm), or resemble dilated cardiomyopathy. However, the absence of LV dysfunction is usually sufficient to distinguish such physiologic ventricular enlargement induced by training from dilated cardiomyopathy.
Arrhythmogenic Right Ventricular Dysplasia (ARVD)
Because highly trained athletes may demonstrate right ventricular enlargement and a variety of depolarization, repolarization and other abnormalities on the ECG, the differential diagnosis between athlete's heart and ARVD may arise. Identification of ARVD by echocardiography is exceedingly difficult because of technical limitations in imaging right ventricular morphology (and assessing right ventricular function), and also because the spectrum of disease is broad. Demonstration of right ventricular or global dysfunction or substantial cavity enlargement supports the diagnosis. Magnetic resonance imaging, however, affords a more definitive noninvasive diagnosis of this condition. In ARVD, ECG's frequently show T wave inversion in V1-V3.
Hypertrophic Cardiomyopathy
The dilemma of distinguishing clinically between athlete's heart and structural heart disease most frequently arises with respect to hypertrophic cardiomyopathy (HCM). While at present there is no single appropriate test that will definitively resolve this question in all such athletes, several are described here that alone or in combination offer a large measure of clarification in most instances for this often compelling differential. The definition of HCM employed here is that of a patient (or athlete ) with evidence of a hypertrophied and nondilated LV in the absence of cardiac or systemic disease that could itself produce hypetrophy of the magnitude present in that individual.
Wall Thickness
In the vast majority of competitive athletes, absolute left ventricular thickness is within normal limits (<12 mm). In some athletes, however, ventricular wall thickness may be more substantial, 13-15 mm, thus raising the possibility of HCM. In patients with HCM, the increase thickness is usually marked; the average wall thickness reported in echocardiographic studies of this disease is approximately 20 mm ranging up to 60 mm. However, an important minority of patients show relatively mild LV hypertrophy with wall thickness values in the range of 13 to 15 mm, and most of these patients are asymptomatic. The diagnostic dilemma may arise in those athletes who fall into this morphological "gray zone" between physiological hypertrophy and maximal wall thickness of 13 or 14 mm, or possibly 15 mm.
In highly trained athletes, the region of predominant LV wall thickness always involves the anterior septum, although the thickness of other segments of the wall are similar (with differences in the range of 1-2mm). In patients with HCM, the anterior portion of the ventricular wall is also usually the region of maximal wall thickening; however, the LV hypertrophy is often heterogeneous, asymmetry is prominent, and occasionally regions other than the anterior septum may show the marked thickening. In addition, contiguous portions of the LV often show strikingly different wall thicknesses in HCM, and the transition between areas is often abrupt.
Diagnosis of HCM in asymptomatic athletes is frequently based on echocardiographic assessment of the magnitude of hypertrophy, on precise quantitative measurements of wall thickness in a single or region of the LV wall. It should be emphasized that, in borderline areas such circumstances present fertile ground for the overt diagnosis.
Since marked increase in LV wall thickness often occurs during adolescence in patients with HCM, young athletes with HCM (<1 may not demonstrate their maximum expression of hypertrophy until physical maturation and development is achieved. Therefore, atheletes with HCM may initially be evaluated with echocardiography where hypertrophy is still only mild or within the borderline range; at that time the differential diagnosis with athlete's heart may be difficult. Such uncertainty can be resolved by serial echocardiographic examinnations which, in months or years, may show more definite wall thickeness confirming the diagnosis of HCM.
Cavity Dimensions
An enlarged LV end-diastolic cavity dimension (>55 mm) is present in morethan one third of highly trained, elite male athletes. Conversely, with HCM, the diastolic cavity dimension is usually small (<45 mm >55 mm only in those who evolve to the end-stage phase of the disease with progressive heart failure and systolic dysfunction. Therefore,in some instances, it is possible to distinguish the athlete's heart from HC on the basis of LV cavity dimension. However, when LV cavity size is between the extremes, this dimension alone will not resolve the correct diagnosis.
Doppler Transmitral Waveform
Abnormalities of LV diastolic filling have been identified using noninvas pulsed Doppler echocardiography or radionuclide angiography in patients with a variety of cardiac diseases associated with LV hypertrophy. Most patients with HCM, including those with relatively mild hypertrophy (i.e., that could be confused with athlete's heart), show abnormal indexes of LV filling independent of whether symptoms or outflow obstruction are present. Typically, the early peak of transmitral flow ("E," due to rapid filling) is decreased and deceleration time of the peak is prolonged; the late peak ("A," due to atrial contraction) is inverting the normal E/A ratio. On the other hand, trained athlete demonstrate normal LV filling patterns. Consequently, in a trained athlete suspected of having HCM, a distinctly abnormal Doppler transmi velocity pattern strongly supports this diagnosis, while a normal pattern is compatible with either HCM or athlete's heart.
Type of Sports Training
The specific nature of athletic training itself has a major influence on the type and magnitude of the changes in LV dimensions. For example study of almost 1000 elite Italian athletes, only about 2% had an thickness of > 13 mm (in the gray zone between physiological hypertrohy and HCM), and this subset was confined to those in rowing sports and cycling. Conversely, most other forms of training, including isometric power) sports such as weight-lifting or wrestling, were not associated with absolute increases in wall thickness beyond 12 mm.
Gender
Gender differences with regard to alterations in cardiac dimensions and mass have been identified in trained athletes. Preliminary findings that highly trained female athletes rarely show LV wall thickness( within the aforementioned gray-zone between athlete's heart and HCM) for example, in a recent report, none of 600 elite women athletes had thickness in the range compatible with the diagnosis of HCM (>13). These observations suggest, therefore, that female athletes with "borderline" left ventricular wall thicknesses of 13-15 mm (in the normal cavity size) are likely to have HCM.
Regression of Hypertrophy with Deconditioning
The observation that increased LV cavity size or wall thickness is a physiological consequences of athletic training may be substantiated by serial echocardiographic examinations showing a decrease in cardiac dimensions and mass with deconditioning. Decrease in wall thickness associated with deconditioning is inconsistent with HCM. Identification of such changes in wall thickness with deconditioning, require: (1) cooperaton from highly motivated competitive athletes to interrupt their training to get serial echocardiographic studies of technical quality.
Familial Transmission and Genetics
The most definitive evidence for the presence of HCM in an athlete with increase in wall thickness probably comes from the demonstration in a relative of HCM. Therefore, in those athletes in whom the distinction HCM and athlete's heart cannot otherwise be achieved definitive echocardiographic screening for affected family members represents a potential method for resolving this diagnostic uncertainty. The absence of HCM in family members, however, does not exclude this disease,which may be "sporadic" (i.e., absent in relatives other than the index person.
Recent advances in defining the genetic alterations responsible for HCM raise the possibility of DNA diagnosis in athletes suspected of having the disease. The genetic abnormalities that cause HCM, however, are heterogeneous. At present, mutations responsible for HCM have identified in 5 genes; cardiac troponin T and I, myosin binding proteins such as Beta-myosin heavy chain and alpha-tropomyosin. This substantiated heterogeneity has made it extremely difficult and time consuming to use techniques of molecular biology for the purpose of clinically resolving differential diagnosis between athlete's heart and HCM.
Author
Barry J. Maron, M.D., is Director of the Hypertrophic Cardiomyopathy Center at the Minneapolis Heart Institute Foundation
Contents of this site are reviewed by The Montgomery Heart Foundation Cardiomyopathy. The information expressed in this web site should not be considered medical advice and individuals should consult their private physician.
Athlete's Heart
The
changes in the heart produced by long-term, intense training
have been the subject of clinical research for a long
time. As new techniques were developed, the research
methods came to include physical examination, chest radiography
and electrocardiography, as well as Holter monitoring,
cardiac catheterization, and echocardiography. However,
even with all of these techniques, there remain unanswered
questions and problems related to the electrocardiograms
recorded from athletes.
Zeppilli[11,12] has
divided the electrocardiographic abnormalities of athletes into three
categories: physiologic changes, which are clearly due to the effects
of training; borderline abnormalities, which may be due to training
but cannot be distinguished from abnormalities due to heart disease;
and abnormalities not due to training, which can nevertheless be observed
in athletes.
The electrocardiographic abnormalities
observed in athletes are listed in Table 13.7. The differentiation
of training-related electrocardiographic abnormalities from those due
to other causes is described in Table 13.8. An example of an electrocardiogram
of a trained athlete is shown in Figure 13.5, and electrocardiograms
of athletes with two types of hypertrophic cardiomyopathy are shown
in Figures 13.6 and 13.7.
The changes in the heart produced by long-term, intense training have been the subject of clinical research for a long time. As new techniques were developed, the research methods came to include physical examination, chest radiography and electrocardiography, as well as Holter monitoring, cardiac catheterization, and echocardiography. However, even with all of these techniques, there remain unanswered questions and problems related to the electrocardiograms recorded from athletes.
Zeppilli[11,12] has divided the electrocardiographic abnormalities of athletes into three categories: physiologic changes, which are clearly due to the effects of training; borderline abnormalities, which may be due to training but cannot be distinguished from abnormalities due to heart disease; and abnormalities not due to training, which can nevertheless be observed in athletes.
The electrocardiographic abnormalities observed in athletes are listed in Table 13.7. The differentiation of training-related electrocardiographic abnormalities from those due to other causes is described in Table 13.8. An example of an electrocardiogram of a trained athlete is shown in Figure 13.5, and electrocardiograms of athletes with two types of hypertrophic cardiomyopathy are shown in Figures 13.6 and 13.7.
Athlete's Heart and Cardiomyopathy
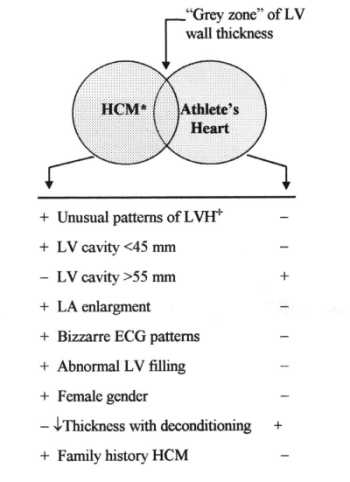
Fig 1: Flow-chart showing criteria used to distinguishing hypertrophic cardiomyopathy (HCM) from athlete's heart when the left ventricular (LV) wall thickness is within the shaded gray zone of overlap, consistent with both diagnoses.
A.
Pelliccia
Istitute of Sport Science,
Department of Medicine
Italian Olympic Committee
Rome, Italy
Long-term athletic conditioning is associated with cardiac morphologic changes, including increased left ventricular (LV) cavity dimension, wall thickness and mass. The extent to which LV dimension is increased in athletes is usually mild: several echocardiographic studies have shown that absolute left ventricular dimensions are increased in athletes in comparison to matched untrained controls by an average of 10% for cavity dimension and 15% for wall thickness. These absolute cardiac dimensions, although increased, usually remain within the accepted upper normal limits, and different in most cases from changes seen in patients with structural cardiac diseases, such as cardiomyopathies.
In elite athletes, however, left ventricular cavity dimensions and, in some instances, wall thicknesses may be markedly increased, well above the upper normal limits predicted by age and body size. Absolute left ventricular wall thickness may exceed > 13 mm, in a range compatible with primary pathologic hypertrophy, i.e. hypertrophic cardiomyopathy in about 2 % of elite athletes. Left ventricular cavity dilatation (end-diastolic transverse diameter may exceed 60 mm, in a range compatible with idiopathic dilated cardiomyopathy in about 15 % of elite athletes. In such circumstances, the morphologic features of the athlete's heart raise the differential diagnosis between an extreme physiologic adaptation to athletic conditioning and a pathologic cardiac condition. This differential diagnosis has implicit ethical, economic and legal implications, because the incorrect identification of a cardiac disease may lead to unnecessary withdrawal of an athlete from competition, thereby depriving that individual of the varied (including economic) benefits of sport. On the other hand, the correct diagnosis of certain cardiovascular diseases may be the basis for disqualification of athlete from competition, in a effort to minimize the risk of sudden cardiac death related to sport activity.
Differential diagnosis of athlete's heart and hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy is a primary cardiac disease, for which the most characteristic morphologic feature is a hypertrophied non-dilated left ventricle in absence of cardiac or systemic disease itself capable of producing left ventricular hypertrophy. The prevalence of this disease in the general population is estimated to be 0.2%. The differential diagnosis of athlete's heart and hypertrophic cardiomyopathy is of crucial importance, because sudden death may be the initial clinical event in young athletes with hypertrophic cardiomyopathy, often in relation to exertion. At present time there is no single approach that will definitively resolve this differential diagnosis in all instances, although several criteria appear useful in this regard, as summarized in Figure 1.
|
| Fig 1: Flow-chart showing criteria used to distinguishing hypertrophic cardiomyopathy (HCM) from athlete's heart when the left ventricular (LV) wall thickness is within the shaded gray zone of overlap, consistent with both diagnoses. |
Left ventricular morphology (Figure 2):
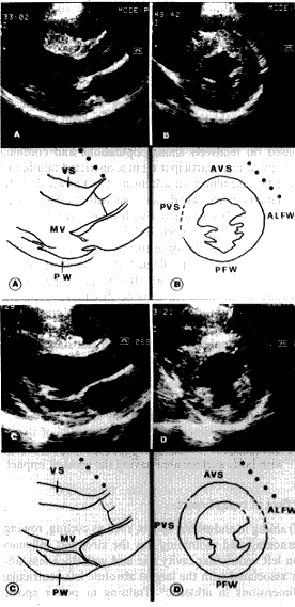 |
Fig. 2. Comparative echocardiographic images of left ventricular hypertrophy characteristics of hypertrophic cardiomyopathy (A, B) and athlete's heart (C, D). Parasternal long axis (A) and short axis (B) views and respective schematic drawings of the left ventricle, at the same calibration, from a 18-year-old with hypertrophic volleyball player with cardiomyopathy. In comparison, the same views and schematic drawings (C, D) from a 25-year-old elite rower with physiologic left ventricular hypertrophy. In the subject with hypertrophic cardiomyopathy, the maximum thickness is 18 mm in the anterior ventricular septum, but the posterior free wall is 8 mm, resulting in a markedly asymmmetric distribution of hypertrophy. In the rower, the maximum ventricular septal thickness is 15 mm, and there is a more symmetric distribution of hypertrophy. The left ventricular cavity is within normal limits (48 mm) in the patient, but is enlarged (58 mm) in the rower. Abbreviations: ALFW = antero-lateral free wall; AVS = anterior ventricular septum; MV = mitral valve; PFW / PW = posterior free wall; PVS = posterior ventricular septum; VS = ventricular septum. |
1) Wall thickening. The
maximum wall thickness found in
highly trained athletes is 15-16 mm, that
likely
represents the upper limit of physiologic
left ventricular wall thickening.
Instead, in patients with hypertrophic
cardiomyopathy,
including those who are asymptomatic
and involved in athletic activities,
the maximum wall thickness shows
a broad range of values, 15 to 60 mm, and
averages
22 mm. A minority of patients with
hypertrophic cardiomyopathy, however, show
relatively
mild hypertrophy (wall thickness
13 to 15 mm) and, therefore, this single
criterion
may not differentiate physiologic
from pathologic hypertrophy in all instances.
2) Distribution of hypertrophy. The distribution of hypertrophy in athlete's heart is sustantially symmetric and regular. Although the different segments of left ventricular wall may not be thickened to an identical degree (maximum wall is usually in the anterior ventricular septum), differences between contiguous segments of left ventricle are generally very small (< 2 mm) and the overall pattern of myocardial hypertrophy appears homogeneous. In patients with hypertrophic cardiomyopathy the distribution of hypertrophy is, in contrast, characteristically asymmetric and heterogeneous.
3) Left ventricular cavity. In athletes with physiologic wall thickening, left ventricular cavity is also consistently enlarged (end-diastolic cavity diameter > 55 mm). The left ventricular cavity shape appears normal, with the mitral valve normally positioned within the cavity and no evidence of left ventricular outflow tract obstruction. In patients with hypertrophic cardiomyopathy, including those who are asymptomatic, left ventricular cavity dimension is small or within normal limits (end-diastolic cavity diameter often < 45 mm. Therefore, in some cases, it is possible to resolve the diagnostic ambiguity of borderline wall thickening in athletes on the basis of left ventricular cavity dimension, when either < 45 or > 55 mm. However, when absolute cavity dimension falls between these two extremes, this criterion does not reliably discriminate between physiologic and pathologic hypertrophy.
4) Dynamic changes in left ventricular hypertrophy. Serial echocardiographic studies have shown dynamic changes in left ventricular wall thickness associated with variations in intensity of training. Of note, in elite and highly-trained rowers examined both at the peak conditioning (when maximum wall thickness averaged 13-15 mm) and after 3 months of deconditioning, was documented a significant reduction in wall thickness (by 2 to 5 mm, mean 3). In hypertrophic cardiomyopathy, no substantial changes in wall thickness would be expected to occur in response to changes in the level of physical activity. Consequently, a brief period of forced deconditioning combined with serial echocardiographic studies may be a useful diagnostic to distinguish physiologic from primary pathologic hypertrophy.
Left ventricular filling
Indexes of left ventricular filling may be useful in distinguishing athlete's heart from hypertrophic cardiomyopathy. Trained athletes with phisiologic LV hypertrophy consistently show normal left ventricular filling pattern In contrast, abnormalities in relaxation and filling have been described as characteristic features of hypertrophic cardiomyopathy, and are present in up to 80% of patient. In hypertrophic cardiomyopathy, diastolic dysfunction is not strictly related to the severity of left ventricular hypertrophy, and may be present in cases with only mild hypertrophy and no symptoms, that most likely require differential diagnosis form athlete's heart. The most frequent abnormalities are a slowed deceleration of early diastolic flow velocity associated with increased late (atrial) peak flow velocity and reversed ratio of early-to-late diastolic peak flow velocity.
Type of sport
Knowledge of the characteristics of athletic training may be helpful in identifying physiologic and pathologic hypertrophy. Marked left ventricular wall thickening is virtually limited to elite, highly-trained athletes engaged in endurance disciplines (primarily rowing, canoeing and cycling). Consequently, absolute increase of left ventricular wall thickness (> 13 mm) in an athlete training in most other sporting disciplines is unlikely to represent the effect of conditioning alone.
Gender
Gender itself may be a useful criterion for discriminating physiologic from pathologic hypertrophy. Physiologic left ventricular wall thickening (>13 mm) is virtually confined to male athletes. On the other hand, men and women with hypertrophic cardiomyopathy do not differ with regard to morphologic expression of the disease, either in terms of maximum wall thickness (mean: 22 mm in both sexes), distribution of left ventricular hypertrophy and number of hypertrophied segments involved. This feature largely reflects the fact that hypertrophic cardiomyopathy is a primary, genetically determined, myocardial disease. Therefore, the finding of borderline wall thickness (i.e., 13-15 mm) in a female athlete is unlikely to be the consequence of athletic conditioning itself and is more likely the expression of pathologic cardiac condition.
Electrocardiogram
In athletes with physiologic left ventricular hypertrophy, a variety of electrocardiographic abnormalities can be found, not uncommonly mimicking those seen in patients with hypertrophic cardiomyopathy, such as markedly increased QRS voltage, T wave inversion and abnormal Q waves. In hypertrophic cardiomyopathy, the 12-lead electrocardiogram is abnormal in the vast majority of patients (> 90 % of cases), showing a wide variety of patterns, which are often bizarre. However, no particular electrocardiographic pattern is specific for the hypertrophic cardiomyopathy and in the individual subject the 12-lead electrocardiogram may not consistently discriminate between athlete's heart and pathologic hypertrophy.
Familial transmission and genetic screening
The most definitive evidence for the presence of hypertrophic cardiomyopathy in an athlete with an increase in wall thickness comes from the demonstration of this disease in a relative. Therefore, echocardiographic screening for affected family members represent a potential method for resolving this diagnostic uncertainty. However, absence of echocardiographic evidence for hypertrophic cardiomyopathy in family members does not exclude occurrence of the sporadic form.
In recent years, a variety of genetic defects have been found in association with familial hypertrophic cardiomyopathy and have raised the possibility of DNA-diagnosis in athletes with borderline hypertrophy. Most of the disease-causing mutations have been identified in genes located on chromosomes 1, 11, 14 and 15; these genes encode the sarcomere proteins cardiac troponin-T, myosin binding protein-C, beta-myosin heavy chain and alfa-tropomyosin, respectively. Furthermore, a large number of mutations for each of these abnormal genes have been described. In most cases, different families have been shown to have different mutations, and some of these have been associated with an unfavourable natural history and clinical course In consideration of the substantial genetic heterogeneity of hypertrophic cardiomyopathy and the relatively complex, time-consuming and expensive techniques necessary for the genetic screening, identification of the disease-causing mutations is, at present, quite laborious and not routinely available for clinical practice.
Differential diagnosis of athlete's heart and idiopathic dilated cardiomyopathy
Idiopathic dilated cardiomyopathy is a primary myocardial disease characterized by left ventricular dilatation and systolic dysfunction. The prevalence of this cardiac disorder has been estimated to be 0.4 % in the general population. Left ventricular cavity dimensions show a broad range of absolute values and in a few instances the degree of dilatation may be minimal. The magnitude of impairment in left ventricular systolic function is also broad, and in the early stages of the disease may be minimal.
On the other hand, left ventricular cavity dimension may be markedly enlarged (end-diastolic transverse diameter > 60 mm) in a sizeable proportion of highly-trained athletes (about 15%). In these individuals, therefore the differential diagnosis between idiopathic dilated cardiomyopathy and physiological left ventricular enlargement of athlete's heart may raise.
Left ventricular morphology (Figure 3)
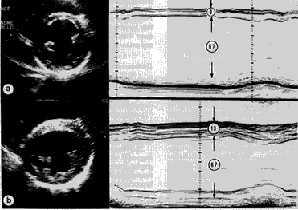 |
Fig. 3. Comparative echocardiographic images of left ventricular dilatation in idiopathic dilated cardiomyopathy (a) and athlete's heart (b). Parasternal, short axis and M-mode tracing of the left ventricle from a 20-year-old asymptomatic patient with idiopatic dilated cardiomyoapthy (a). In comparison, the same views at the same calibration, from a 26-year-old elite rower, who was a participant at the Olympic Games (b). In both the patient and the elite athlete, the left ventricular cavity is dilated to the same extent (67 mm); however, the ventricular septum and free wall are relatively thin (8 mm) and show a reduced systolic motion in the patient, but are increased (up to 13 mm) and show a normal systolic motion in the rower. In the patient, the mitral valve and papillary muscles are superiorly located; in the athlete, the mitral valve is normally located within the left ventricular cavity. |
In athletes, physiologic left ventricular cavity enlargement is associated with enlargement of the right ventricular and atrial chambers, as an expression of a global cardiac remodeling. In elite athletes, the maximum left ventricular end-diastolic cavity dimension does not exceed 70 mm, that likely represents the upper limit of physiologic left ventricular enlargement. In patients with dilated cardiomyopathy, on the other hand, dilatation of both ventricles is common, but left ventricular enlargement usually predominates and may be substantial, as an expression of primary myocardial disease. The enlarged left ventricular cavity in athletes maintains the normal ellipsoid shape, while LV cavity in patients with dilated cardiomyopathy usually achieves a more spherical shape, in association with impaired contractility and deterioration of the clinical status. Indeed, in dilated cardiomyopathy, mitral regurgitation is common, due to dilatation and distortion of the mitral ring. Left ventricular wall thickness may be within normal range in both instances, but relative wall thickness is usually (mildly) increased only in the athlete’s heart.
Left ventricular function
In athletes with physiological left ventricular dilatation, global systolic function is normal, and regional wall motion abnormalities are absent. Therefore, in an athlete with left ventricular cavity dilatation, evidence of systolic dysfunction is the most reliable criterion for differentiating athlete's heart from primary pathologic condition, such as idiopathic dilated cardiomyopathy.
Type of sport
In assessing whether an enlarged left ventricular cavity in an athlete represents a physiological or pathologic condition, knowledge of the athlete's training may also be useful. Long-term, intensive training in largely aerobic disciplines (primarily cycling, cross-country skiing, canoeing, rowing and soccer) has been shown to represent the strongest determinant for p hysiologic enlargement of left ventricular cavity.
Electrocardiogram
A wide range of electrocardiographic alterations have been described in association with both physiologic left ventricular dilatation and idiopathic dilated cardiomyopathy. However, no particular pattern is specific for idiopathic dilated cardiomyopathy and, in the individual athlete, the analysis of 12-lead electrocardiogram may not reliably discriminate between athlete's heart and this pathologic condition.
* Assumed to be the nonobstructive form of HCM in this discussion, since the presence of substantial mitral valve systolic motion would confirm, per se, the diagnosis of HCM.
+ May involve a variety of abnormalities, including heterogeneous distribution of left ventricular hypertrophy (LVH) in which the asymmetry is prominent, and adjacent regions may be of greatly different thicknesses, with sharp transition evident between segments; also, patterns in which the anterior ventricular septum is spared from the hypertrophic process and the region of predominant thickening may be in the posterior portion of the septum or anterolateral or posterior free wall.
Co-Authors:
F.M. Di Paolo
R. De Luca
Istitute of Sport Science, Department of
Medicine
Italian Olympic Committee, Rome, Italy