The prevalence, presentation, clinical significance, and long -
term implications of atrial fibrillation depend heavily upon
the clinical circumstance in which it occurs. Among the cross-sectional
studies of prevalence, there is a large gradient across age
categories, ranging from less than 0.5% in young adults to the
range of 1 to5% through the decades from 40 to 70 years and
reaching rates in excess of 10% in some beyond age 70. At each
age, however, prevalence is powerfully influenced by the presence
of disease, especially rheumatic mitral valve disease, but also
nonrheumatic abnormalities.
The clinical presentation ranges ranges from a minimally symptomatic
or asymptomatic incidental finding to acute pulmonary edema (fluid
in the lungs) in patients with advanced mitral or aortic stenosis. Between
these extremes atrial fibrillation may herald the presence of
noncardiac disorders (e.g., thyrotoxicosis), alert to the significance
of another cardiac disorder (e.g., Wolff-Parkinson-White syndrome), constitute
a transient complicating factor of another cardiac disorder (e.g.,
acute myocardial infarction or systemic arterial hypertension),
or occur as an isolated event having no inherent significance (e.g.,
lone paroxysmal atrial fibrillation in healthy young adults).
The hemodynamic consequences of artial fibrillation are due
to two factors:
(1) the loss of atrial systole may impair ventricular
function in the noncompliant ventricle (e.g., aortic stenosis, left
ventricular hypertrophy) or the dilated ventricle with systolic
dysfunction and
(2) a rapid ventricular rate encroaches upon
diastolic filling of the left ventricle and the coronary arteries.
The risk of of embolism (dislodgement of heart chamber blood
clot, which goes to the brain and blocks a blood vessel) and
a resultant stroke is a long-term concern of special importance. Atrial
fibrillation may occur in paroxysmal, persistent, and chronic
patterns.
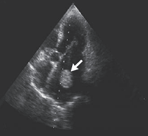
Figure 15b-1: Calcified
left atrial thrombus.
From J.M.Pappachan,M.D.
and B.C. Bino M.D., Calcified Left Atrial Thrombus; Images in
Clinical Medicine ,Volume 356:e9, New England Journal Medicine,
March 8, 2007. |
|
|
|
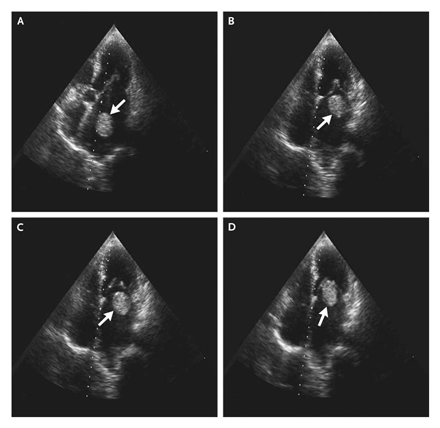
Figure 15b-2: Above thrombus in various positions(4) in left atrium due to
contraction of cardiac muscle. |
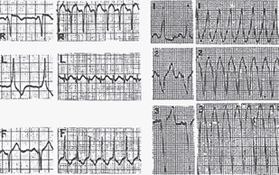
Atrial
fibrillation is an arrhythmia, characterized by grossly disorganized
atrial electrical activity, which is irregular in respect to
both rate and rhythm (see figures 14,
15a,
15b).
Atrial
fibrillatory waves are best seen in leads V1 and usually clearly
evident in II, III, AVF of the EKG. The waves
are large,
coarse or almost imperceptible (see figure 14).
In absence of discernible atrial electrical activity, a grossly
irregular ventricular rhythm still suggest the presence of atrial
fibrillation. Coarse atrial fibrillation waves (f) may be confused
with atrial flutter, but the irregular ventricular response
is helpful in making the distinction (see figure
15a).
In
contrast, obvious, coarse flutter waves (really "f"
waves) with a regular ventricular response, especially if slow,
suggest the coexistence of high grade AV block with atrial fibrillation.
| Origin
of atrial fibrillation |
1). A discharge from a single rapidly firing focus (or foci),
usually located in or near the pulmonary veins can precipitate
and perpetuate this tachycardia (see figure
11h). These foci may mimic the appearance of atrial fibrillation
on the surface electrocardiogram or, more commonly, may degenerate
or trigger classic atrial fibrillation. Repeated episodes of
this arrhythmia can result in a marked shortening of the atrial
refractory period and a loss of the normal lengthening of atrial
refractiveness at slower heart rates. This phenomenon called
atrial remodelling may be reversible with maintenance of sinus
rhythm.
Pretreatment
with verapamil, a calcium channel blocker, may markedly reduce
the extent of remodelling, suggesting that cytosolic calcium
overload is a contributory factor. The use of verapamil in conjunction
with antiarrhythmic drugs before cardioversion may reduce the
risk of recccurence of arrhythmia.
2). Multiple, small wavelets (circuits)
of reentry are involved in most cases of atrial fibrillation,
arising constantly in the atria, which are diseased by fibrosis
and/or inflammation (see
animation, figure 159).
These circuits collide, become extinguished and arise again.
The" life-time" of each individual wavelet is rather
short; within either the left or right atrium, a whirling wavelet
seldom survives a period of longer than a few hundred milliseconds.
It is crucial to the perpetuation of atrial fibrillation that
each atrium gets a constant supply of "new" impulses
from the other atrium.
A
critical mass of atrial tissue is required to sustain the minimal
number of simultaneous circuits necessary for the perpetuation
of the arrhythmia. The critical number of wandering wavelets
for perpetuation of fibrillation is higher than three and less
than or equal to six. The number of wavelets at any given moment
is determined by the balance between the disappearance of "old
" wavelets and the genesis of new "ones". Dying
out of existing wavelets can be caused by fusion or collision
with another wavelet, by reaching the border of the atrium,
and because the advancing depolarization wave meets an area
where the myocardium has not recovered its excitability from
a foregoing activation.
New
wavelets are formed by the division of an existing wave at a
local area of conduction block, an offspring traversing toward
the other atrium, and possible sources of impulse formation
(see animation, figure 160).
The multiple fragmented wavelets, which continuously change
their width and direction and constantly travel in only partially
recovered tissue, are propagating with highly varying velocity.
The
two above explanations for fibrillation are not necessarily
mutually exclusive. It is quite conceivable that in cases in
which fibrillation continues for many years, both mechanisms
act together.
Reference:Alessie,A.
and others,Experimental Evaluation of Moe's Multiple Wavelet
Hypothesis of Atrial Fibrillation,Zipes,D.P. and Others,Cardiac
electrophysiology and Arrhythmias;Orlando,Fa.,Grune and Stratton,1985,265-75.
Triggers
that may initiate the arrhythmia include changes in autonomic
tone (see definition autonomic
nervous system), acute or chronic changes in in atrial wall
tension, atrial ectopic foci (including foci from pulmonary
veins), and local factors.
Drugs
can prevent atrial fibrillation by increasing the circuit wavelength,
and invasive techniques can prevent it by decreasing the size
of the atrial segments.
For
example, the maze procedure (requiring cardiopulmonary bypass)
consists of the excision of the atrial appendages, isolation
of the pulmonary veins, and creation of a narrow, tortuous path
of atrial tissue by carefully placed incisions, which directs
the sinus node impulses across the atria to the atrioventricular
node. The incisions are placed so that no area is wide enough
to sustain multiple reentry circuits, and thus atrial fibrillation
cannot occur. Several dead-end alleyways create a maze-like
pathway and permit the depolarization of all the atrial tissue.
There
is evidence that minimally invasive surgery and cryoablation
can be used to accomplish the maze procedure in the beating
heart without cardiopulmonary bypass.
Reference:Falk,R.H.,Atrial
Fibrillation,N Engl J Med,Vol.344,No.14,April5,2001,1067-1078.
The
fact that a single focus can definitely cause some forms of
atrial fibrillation facilitates catheter ablation to eliminate
the arrhythmia and thus, offer a cure for some patients (see
figure 11e). Patients with this type of atrial fibrillation
are frequently young with structurally normal hearts, have paroxymal
episodes, and the EKG often often shows bursts of an atrial
tachycardia or frequent premature atrial complexes, sometimes
initiating the atrial fibrillation.
The
atrial focus is most commonly located in the pulmonary veins
(figures 104b,
105a), more often in the upper than in the lower (see
figure 11g), and can be ablated by transseptal techniques
(passing special catheters and guidewires across the interatrial
septum into the left atrium and on into the pulmonary veins
and delivering radiofrequency energy to the suspected pulmonary
vein focus).
The ostia of the pulmonary veins are explored
to 15mms. into the vessel from the ostia to prevent stenosis,
where the radiofrequency ablation is applied (see
figures 11a, 11b, 11c)
Reference:Haissaguerre M. and others,Catheter ablation of chronic
atrial fibrillation targeting the reinitiating triggers,J.Cardiovasc.
Electrophysiol.2000;1112-10).
The P-wave morphology of the PAC or atrial
tachycardia from the pulmonary vein can be used to help locate
the responsible pulmonary vein for the atrial fibrillation.
The
site of focal atrial fibrillation can be predicted from close
analysis of mapping the sequence of coronary sinus activation
and its relationship to right atrial eletrograms, which may
limit the mapping of all the pulmonary veins. It has been found
that left upper and lower PV pacing is associated with early
activation of distal coronary sinus (CS). Left upper PV pacing
is associated with high right atrial activation (HRA) and HIS
atrial activation prior to proximal CS. Pacing of right sided
PVs is associated with proximal to distal CS activation. Right
upper PV pacing is associated with early activation of HRA prior
to HIS and CS activation. Right lower PV pacing is associated
with HIS atrial activation prior to HRA.
Reference:Zipes
Douglas,MD, Journal of American College of Cardiology,vol.36,no.6,2000,Nov.15,pp.1746-8l.
Reference:Davendra,M.
AND OTHERS,JACC, Abstracts,Cardiac Arrhythmias, Prediction of
Site of Focal Atrial Fibrillation from Sequence of Coronary
Activation and its Relationship to Right Atrial Electrograms,Feb.2001,p.118a
Atrial
fibrillation may be minimally symptomatic to asymptomatic, or
associated with fatigue, palpitations, nonspecific symptoms,
reduced quality of life, reduced memory in elder patients, acute
pulmonary edema (lungs full of fluid with severe shortness of
breath) occurring in mitral stenosis or aortic stenosis.
It
may be associated with thyrotoxicosis (excess thyroid hormone
in the blood stream), WPW syndrome (an atrial tachycardia),
hypertension, complication of acute myocardial infarction (heart
attack).
It may also be of no significance.
See below for continuation.
Prevalence in young adults is less than
0.5%, but is 1 to 5% in ages 40 to 70, and over 10% in those
over 70 years.
The incidence is influenced by the presence of mitral valve
disease.
It can be caused by noncardiac disorders
like thyrotoxicosis (excessive levels of throid hormone in the
blood) as well as other cardiac disorders like WPW syndrome
(see figure 3,3a), acute myocardial
infarction(see definition electrocardiogram on this website,figures94-2,94-6,94-7,94-8
table 1), and hypertension (high blood pressure, see definition"
hypertension" on this website). It can occur even in healthy
people.
 |
|
|
The consequences include loss of atrial
systole (contraction)causing a decrease in ventricular stroke
volume of up to 20% and rapid irregular ventricular rates with
wide swings, risk of embolism (dislodgement of blood clots formed
in the heart chambers with dissemination into other arteries
through the body) and stroke. The tachycardia can cause in ultrastructural
changes that cause a reversible ventricular dysfunction.
EKG features (see figures
14,
15a,
15b): The ventricular response is irregular, whereas in atrial
flutter it is regular.
| Evaluation
of First Episode of Atrial Fibrillation |
A thorough investigation is necessary
to determine the cause:
1) Primary electrical cause
2) Hemodynamic abnormalities
3) Systemic abnormalities like thyrotoxicosis
4) Unrecognized heart disease must be ruled out (echocardiography
is invaluable for evaluating cardiac abnormalities).
5) Pulmonary embolism and thyroid disease (detected with thyriod
function tests) must be considered
6) If no etiology is found, then lone atrial fibrillation
carries a good prognosis.
Chronic lone atrial fibrillation could indicate
a higher risk. However, excessive cigarettes, alcohol and/or
coffee consumption, stress, and fatigue may be causative factors.
If
organic heart disease is found, it should be treated appropriately.
| Management
of Short-duration Paroxymal Atrial Fibrillation |
In
the absence of heart disease, episodes of A.F. of less than
48 hours are managed conservatively. Rest, mild sedation, adding
digoxin, intravenous(iv) diltiazem, iv beta-blocker, or some
combination for control of the ventricular rate is an acceptable
approach (see tables 1 and 2).
Although the atrial rate usually exceeds 350
beats/min., the mean resting ventricular rate in a patient with
atrial fibrillation of new onset is between 110 and 130/min.
In patients with the Wolff-Parkinson-White syndrome and a short
refractory period of the accessory pathway, the ventricular
response may exceed 250/min. In such cases, the electrocardiogram
demonstrates a wide-complex QRS tachycardia, due to predominant
accessory pathway conduction (see figure 3). A resting ventricular
rate higher than 150/min. in the absence of preexcitation should
raise the suspicion of a hyperadrenergic state such as occurs
in thyrotoxicosis, fever, or acute gastrointestinal bleeding.
A slow rate in the absence of medication may occur with high
vagal tone in young athletes or in patients with conduction-system
disease.
If
heart disease is present and especially if the hemodynamic (stiff
ventricles with low output due to heart muscle weakness, and
mitral valve disease and/or coronary atherosclerosis) condition
require either the mechanical benefit of atrial systole or a
slow ventricular rate for adequate diastolic filling of the
ventricles, immediate reversion to a sinus rhythm or slowing
of the ventricular rate may be mandatory.
Immediate cardioversion (electrical shock
to the chest overlying the heart) may be needed, if there are
signs of heart failure. If the patient is stable, digoxin, intravenous
(IV) verapamil (beta-blocker, medication), iv diltiazem, or
procainamide (antiarrhythmic drug) or some combination may be
tried (see table 1 and 2).
Even
if their condition is clinically stable, patients with atrial
fibrillation and a wide-complex (QRS) ventricular response related
to the preexcitation syndrome should also be considered for
early electrical cardioversion, since the response to antiarrhythmic
agents is unpredictable in such patients and most agents used
for control are contraindicated.
In
the absence of underlying heart disease long term pharmacologic
therapy may be used to prevent recurrence (see table 2).
In
trials of anticoagulant therapy, patients with paroxysmal atrial
fibrillation had the same risk as subjects with persistent atrial
fibrillation. Thus, unless a patient with paroxysmal arrhythmia
is younger than 65 years old and has no hypertension or underlying
herat disease, long term warfarin therapy should be instituted.
Antiarrhythmic-Drug
Therapy
Several
drugs have been shown to be effective in the treatment of paroxysmal
atrial fibrillation, including propafenone, flecainide, and sotalol
(see table 2). These agents often do not totally abolish the
arrhythmia, but the increase the length of the interval between
the paroxysms and reduce the symptoms but not necessarially
the risk of thromboembolism. Also, patients with symptomatic
arrhythmia may have numerous episodes of asymptomatic atrial
fibrillation, which pose a risk of thromboembolism.
Use
of antiarrhythmic-drugs by slowing the heart rate can convert
symptomatic episodes to asymptomatic ones, but the risk of a
stroke still remains. Holter monitoring (see definition of
Holter monitoring) may be helpful inassessing these episodes.
Reference:Falk,R.H.,Atrial
Fibrillation,N Engl J Med,Vol.344,No.14,April5,2001,1067-1078.
| Management
of Persistent Atrial Fibrillation |
Once
an episode of atrial fibrillation has lasted more than seven
days, spontaneous conversionis rare and the condition is defined
as persistent. Restoration of sinus rhythm has to balanced against
relief of symptoms if present versus the likelihood of side
effects, especially proarrhythmia. Pharmacologicconversion is
successful in 10 to 30 % of cases, depending on the duration
of the arrhythmia and the drug used.
So
synchronized, direct-current cardioversion is usually required
in order to restore sinus rhythm. This involves using at least
300 J of energy with most defibrillators currently in use. But
the recent introduction of defibrillators with a biphasic wave
form, rather than the traditional monophasic damped-sine wave
form, is associated with a marked decrease in the energy rquired
for atrial fibrillation and with fewer failures.
Failure
to terminate an arrhythmia with a specific arrhythmic agent
does not mean that the same drug will be ineffective in maintaining
sinus rhythm after electrical cardioversion.
In
some cases, sinus rhythm is not restored or is restored only
briefly by electical cardioversion. In such a case, the use
of intravenous ibutilide followed by another shock increases
the likelihood of restoration and maintenance of sinus rhythm.
But ibutilide has to be used with caution in patient with impaired
ventricular function, since it may cause torsade de pointes
(see defintion ventricular
tachycardia) and the safety of this approach in patients
already receiving another antiarrhythmic drug has not been established.
If a patient fails to return to sinus rhythm even for one or
two beats despite these measures, transvenous internal cardioversion
may be successful.
Amiodarone
has been shown to be superior to both sotalol and propafenone
for the maintenance of sinus rhythm. To date, only dofetilide
and amiodarone have been shown not to increase mortality when
prescribed to patients with heart failure.
Reference:Falk,R.H.,Atrial
Fibrillation,N Engl J Med,Vol.344,No.14,April5,2001,1067-1078.
Heart-rate
control
The aims of pharmacologic control of the heart
rate in patients with persistent atrial fibrillation are to
minimize symptoms related to swings in heart rate and prevent
excessive tachycardia during normal daily activities. Digoxin
may be acceptable as the sole therapy in an elderly, sedentary
patient, but ir is not very effective for preventing excessive
tachycardia during moderate exertion. Beta-blocking agents,
verapmil, and diltiazem are much more effective, and there is
synergism between these drugs and digoxin. Beta-blocking agents
are probably the drugs of choice in patients with atrial fibrillation
and coronary artery disease, and they may also be valuable when
systolic dysfunction is present.
Reference:Falk,R.H.,Atrial
Fibrillation,N Engl J Med,Vol.344,No.14,April5,2001,1067-1078.
If
elective cardioversion is to be used, 3 weeks of anticoagulation
(use of coumadin (warfarin) to lower prothrom time, a measure
of a protein produced by the liver) should proceed the procedure
(to prevent blood clots and emboli) and should be continued
until sinus rhythm has been maintained for four weeks after
cardioversion.
An
alternative approach has recently been suggested in patients
who have been shown by transesophageal echocardiography(TEE)
to be free of thrombi in the left atrium and its appendage (figures
146,
147,
148).
They receive short term anticoagulant therapy with the use of
low molecular weight heparin (as outpatients), which can serve
as a bridge therapy to warfarin (versus intravenous unfractioned
heparin for inpatients for 4-5 days as bridge therapy to warfarin).
There
are certain subgroups that may benefit from the TEE-guide strategy.
First, the inpatientwith new-onset AF (< 4 weeks duration),
regardless of risk profile, may benefit from early cardioversion
using the TEE strategy. This may be particularly important for
high risk patients (such as those with congestive heart failure,
previous embolism or hemodynamic instability) in whom the prompt
return of normal sinus rhythm would be beneficial.
Second,
the high risk patient may benefit from further risk stratification
by TEE to identify left atrial appendage thrombus, severe spontaneous
echocardiographic contrast or complex atheroma (figures 146,
147, 148). The identification of a thrombus before cardioversion
would lead to cancellation of the cardioversion and more prolonged
anticoaglation therapy.
Third,
even for patients in whom the likehood of thrombus is low, eliminating
the need for prolonged anticoagulation pre-cardioversion by
ruling out the presence of thrombus would allow early cardioversion
and avoid the delay for return of sinus rhythm.
Reference:Klein,A.L.,and others,Role
of TEE-Guided Cardioversion of Patients With AF,JACC,Vol.37,No.3,2001,pp.691-701.
In a recent study of 1222 patients with atrial
fibrillation of more than two days duration assigned to either
treatment guided by findings on TEE or conventional therapy,
it was found that there was no significant difference between
the two groups in the rate of embolic events.But the rate of
hemorrhagic events was significantly lower in the TEE group
(apparently due to the duration of anticoagulation in the conventional
group being almost double that with the other approach allowing
more opportunity for bleeding), who also had a shorter time
to cardioversion and a greater rate of successful restoration
of sinus rhythm. At eight weeks, there were no significant differences
between the two groups in the rates of death or maintenance
of sinus rhythm or in functional status. Thus, the strategy
of using TEE to guide treatment may be considered a safe and
clinically effective alternative to the conventional treatment
strategy.
Reference: Klein,A.L.,and others,USE OF
TEE TO GUIDE CARDIOVERSION IN PATIENTS WITH ATRIAL FIBFILLATION,N
ENGL MED,VOL.344,NO.19,MAY10,2001,1411-1420.
If
cardioversion is not attempted, and there are recurring episodes
of atrial fibrillation, then long term anticoagulation with
coumadin (warfarin,a drug which lowers the prothrombin
protein to prevent coagulation) is indicated to prevent
emboli.
| Management
of Chronic Atrial Fibrillation |
Pharmacologic
or electrical cardioversion has not had a high rate of success.
Usually only one attempt is made to convert with electrical
cardioversion (1/3 will revert back to A.F.).
Catheter modification of the AV juncture
(AV node) or complete ablation of the AV junction with permanent
pacing may provide heart rate control.
A
recent study reports that in the absence of underlying heart
disease, survival among patients with atrial fibrillation after
ablation of the artioventricular node is similar to expected
survival in the general population. Long term survival is similar
for patients with atrial fibrillation, whether they receive
ablation or drug therapy. Control of the ventricular rate by
ablation of the atrioventricular node and permanent pacing does
not adverselly affect long term survival.
See
use of radiofrequency ablation in cases of atrial fibrillation
originating in the pulmonary veins described above.
Zipes
Douglas,MD,Journal of American College of Cardiology,vol.36,no.6,2000,Nov.15,pp.1746-8l.
Ozcan,C. and others, LONG TERM SURVIVAL
AFTER ABLATION OF THE ATRIOVENTRICULAR NODE AND IMPLANTATION
OF A PERMANENT PACEMAKER IN PATIENTS WITH ATRIAL FIBRILLATION
NEng J Med,Vol.344,No.14,April5,2001.
Anticoagulation
(use of medicines like warfarin or heparin to prevent coagulation
of blood to a degree to prevent blood clots from forming in
the heart and other vessels).
This
is very important in the management of patients with persistent
or chronic atrial fibrillation, since patients with atrial fibrillation
have a five fold increase in risk of stroke from embolism (blood
clots thrown from heart to other vessels in the body).
Prolonged
episodes of atrial fibrillation frequently cause mechanical
dysfunction of the atrium. Restoration of sinus rhythm is generally
associated with the normalization of function over a period
of two to four weeks. Possibly because of the delayed recovery
of atrial mechanical function, the risk of thromboembolism posed
by atrial fibrillation seems to persist for a few weeks after
cardioversion.
In
rheumatic heart disease, the risk increases to 17 times a control
group. Other high risk groups include dilated hearts due to
cardiomyopathy, dilated left atrium, atrial fibrillation of
recent onset, history of prior embolism, left ventricular wall
increase thickness (hypertrophy), thyrotoxicosis, greater than
60 years of age, and prior to elective electrical cardioversion.
Bleeding
is a risk when on long term anticoagulation therapy. Intracranial
bleeding is one of its major complications.
|