M-mode
echocardiogram showing moderate pericardial effusion
present anteriorly(PE) and posteriorly(PPE).
RVW=right ventricular wall; IVS=interventricular
septum; endo=endocardium; epicardium; MV=mitral
valve; LA=left atrium.
(R.Shabetai:The
Pericardium.New YORK ,Grune&Stratton,1981,modified)
|
This is a syndrome in which there is an accumulation of fluid
in the pericardial sac (see illustration). It may be caused
by acute pericarditis, tumors, especially the bronchogenic,
mammary, or lymphomatous types, post-radiation, and post-trauma.
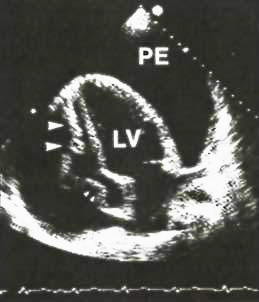
Two-dimenional
apical four-chamber view from a patient with cardiac
tamponade.This diastolic frame shows a large pericardial
effusion(pe) that completely surrounds the heart.The
right ventricular cavity is virtually nonexitent.There
is both right ventricular wall collapse (large arrows)
and right atrial wall collapse(small arrows consistent
with tamponade.
LV=left
ventricle.
Hurst'sThe
Heart,8th edition,p.402
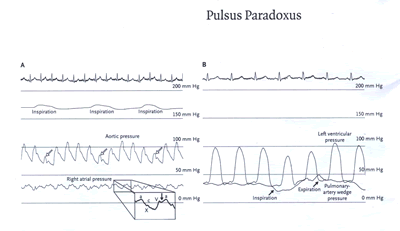
A 45-YEAR-OLD WOMAN WITH A HISTORY OF HODGKIN'S LYMPHOMA
that had been treated with mantle-field irradiation
20 years earlier presented with pleuritic chest pain,
progressive dyspnea, and presyncope. Notable findings
on physical examination included tachycardia, a systolic
blood pressure of 100 mm Hg with pulsus paradoxus (a
20 mm Hg decrease in systolic pressure on inspiration),
an elevated jugularvenous pressure (15 cm ofwater),
and a three-component cardiac friction rub. The electrocardiogram
showed sinus tachycardia and low voltage. An echocardiogram
showed a small, circumferential pericardial effusion
that could not be approached safely by pericardiocentesis.
The patient subsequently underwent cardiac catheterization.
Panel A shows the electrocardiogram, the respirogram,
and the tracings of aortic pressure and right atrial
pressure. There was an elevated right atrial pressure
with an X descent but blunting of the Y descent (solid
arrow). On inspiration, there was a 30 mm Hg decrease
in aortic systolic pressure as well as a decrease in
pulse pressure (open arrows) -findings that constitute
pulsus paradoxus. The tracings of left ventricular pressure
and pulmonary-artery wedge pressure (Panel B) show that
the pulsus paradoxus is caused by underfilling of the
left ventricle during inspiration (due to a drop in
the initial pressure gradient between the pulmonary-artery
wedge pressure and the left ventricular diastolic pressure).
The patient underwent surgery, and a tense, inflamed
pericardium was noted. To relieve the pericardial tamponade,
500 ml of serosanguineous fluid that was under pressure
was drained from the pericardial space, and a complete
pericardiectomy was performed.
Lambert A. Wu, M.D.,Rick A. Nishimura, M.D.
Mayo Graduate School of Medicine Rochester, MN 55905,NEJM349;7;Page
666,August14,2003.
ACUTE CARDIAC TAMPONADE
Cardiac tamponade is life-threatening,slow or rapid
compression of the heart due to the pericardial accumulation
of fluid, pus, blood, clots, or gas, as a result of
effusion, trauma, or rupture of the heart. Because the
causes of pericardial disease and thus of tamponade
are diverse, clinicians must choose the most probable
diagnosis, always anticipating surprises. Thus, traumatic
tamponade is most apt to follow cardiac surgery, and
tuberculous tamponade is relatively common in Africa
but rare in the United States.
Understanding the physiological changes produced by
tamponade is essential to diagnosis and treatment. The
primary abnormality is rapid or slow compression of
all cardiac chambers as a result of increasing intrapericardial
pressure. The pericardial contents first reach the limit
of the pericardial reserve volume - the volume that
would just distend the pericardium - and the rate of
expansion then increases, soon exceeding that of pericardial
stretch. Although the pericardium stretches normally
over time, at any instant it is inextensible, making
the heart compete with the increased pericardial contents
for the fixed intrapericardial volume. As the chambers
become progressively smaller and myocardial diastolic
compliance is reduced, cardiac inflow becomes limited,
ultimately equalizing mean diastolic pericardial and
chamber pressures. Key elements are the rate of fluid
accumulation relative to pericardial stretch and the
effectiveness of compensatory mechanisms. Thus, intrapericardial
hemorrhage from wounds or cardiac rupture occurs in
the context of a relatively stiff, unyielding pericardium
and quickly overwhelms the pericardial capacity to stretch
before most compensatory mechanisms can be activated,
whereas in the case of a slow increase in pericardial
volume as a result of inflammation, 2 liters or more
may accumulate before critical, life-threatening tamponade
occurs.
The stiffness of the pericardium determines fluid increments
precipitating tamponade, as illustrated by characteristic
pericardial pressure-volume (strain-stress) curves (cardTamp-Fig.
1): there is an initial slow ascent, followed by an
almost vertical rise. This steep rise makes tamponade
a "last-drop" phenomenon: the final increment
produces critical cardiac compression, and the first
decrement during drainage produces the largest relative
decompression.
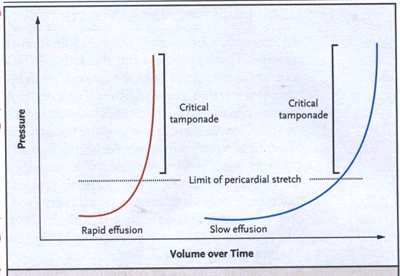
cardTamp-Fig. 1. Cardiac Tamponade.
Pericardial pressure-volume (or strain-stress) curves
are shown in which the volume increases slowly or rapidly
overtime. In the left-hand panel, rapidly increasing
pericardial fluid first reaches the limit of the pericardial
reserve volume (the initial flat segment) and then quickly
exceeds the limit of parietal pericardial stretch, causing
a steep rise in pressure, which becomes even steeper
as smaller increments in fluid cause a disproportionate
increase in the pericardial pressure. In the right-hand
panel, a slower rate of pericardial filling takes longer
to exceed the limit of pericardial stretch, because
there is more time for the pericardium to stretch and
for compensatory mechanisms to become activated.
The true filling pressure is the myocardial transmural
pressure, which is intracardiac minus pericardial pressure.
Rising pericardial pressure reduces and ultimately offsets
this transmural pressure, first for the right heart
and ultimately for all chambers. On average, during
inspiration and expiration, the right heart increases
its filling at the expense of the left, so that its
transmural pressure transiently improves and then reverts
during expiration. In florid tamponade such a mechanism
cannot compensate for reduced stroke volumes, since
these volumes depend on the elements that protect cardiac
output and arterial pressures, principally beta-adrenergically
increased heart rate, peripheral resistance and ejection
fractions, and given sufficient time, expansion of the
blood volume. Additional compensation provided by neurohormonal
stimulation is similar to that occurring in heart failure,
except that the levels of atrial natriuretic peptide
do not increase because the compressed myocardium
cannot stretch.
Acute tamponade thus reflects decompensation as patients
reach the steep portion of the pressurevolume curve
(cardTamp-Fig. 1). Moreover, intercurrent factors can
cause the decompensation of any effusion - for example,
the influx ofblood, effusion-expanding osmotic effects
of fragmenting intrapericardial clots, or inflammatory
stiffening of the pericardium. Finally, although coronary
blood flow is reduced in tamponade, there is no ischemic
component because coronary flow remains proportional
to the reduced work and operational requirements of
the heart.
CLINICAL FINDINGS
Critical tamponade is a form of cardiogenic shock,
and the differential diagnosis may initially be elusive.
Since most symptoms are nonspecific, tamponade must
be suspected in many contexts - for example, in patients
who have wounds of the chest or upper abdomen and hypotension
or in those who have hypotension preceded by symptoms
of an inciting pericardial disease, such as chest discomfort
and pleuritic pain. Tachypnea and dyspnea on exertion
that progresses to air hunger at rest are the key symptoms,
but it may not be possible to obtain such information
from patients who are unconscious or obtunded or who
have convulsions at presentation. Most patients are
weak and faint at presentation and can have vague symptoms
such as anorexia, dysphagia, and cough. The initial
symptom may also be one of the complications of tamponade,
such as renal failure.
Most physical findings are equally nonspecific. Tachycardia
(a heart rate of more than 90 beats per minute) is the
rule. Exceptions include patients with bradycardia during
uremia and patients with hypothyroidism. Contrary to
common belief, a pericardial rub is a frequent finding
in patients with inflammatory effusions. Heart sounds
may be attenuated owing to the insulating effects of
the pericardial fluid and to reduced cardiac function.
Although the precordium may seem quiet, an apical beat
is frequently palpable, and patients with preexisting
cardiomegaly or anterior and apical pericardial adhesions
may have active pulsations.
Clinically significant tamponade usually produces absolute
or relative hypotension; in rapid tamponade, patients
are often in shock, with cool arms and legs, nose, and
ears and sometimes peripheral cyanosis. Jugular venous
distention is the rule, with peripheral venous distention
in the forehead, scalp, and ocular fundi unless the
patient has hypovolemia. Thus, rapid tamponade, especially
acute hemopericardium, may produce exaggerated jugular
pulsations without distention, because there is insufficient
time for blood volume to increase. Venous waves usually
lack the normal early diastolic y descent. In compressive
pericardial disease (tamponade and constriction), venous
waves are not outward pulsations; rather, x and y collapse
from a high standing pressure level.
A key diagnostic finding, pulsus paradoxus-conventionally
defined as an inspiratory systolic fall in arterial
pressure of 10 mm Hg or more during normal breathing-is
often palpable in muscular arteries. With very low cardiac
output, however, a catheter is needed to identify pulsus
paradoxus. Other conditions causing pulsus paradoxus
include massive pulmonary embolism, profound hemorrhagic
shock, other forms of severe hypotension, and obstructive
lung disease. Moreover, certain conditions can impede
the identification of tamponade by making pulsus paradoxus
undetectable (Table 1).
Table 1. Conditions Leading to the Absence of Diagnostic
Pulsus Paradoxus in Cardiac Tamponade.
|
| Condition |
Consequence |
| Extreme hypotension, as in shock, and even severe tamponade |
May make respiration-induced pressure changes unmeasurable |
| Acute left ventricular myocardial infarction with occasional
effusion causing tamponade |
|
| Pericardial adhesions, especially over the right heart |
Volume changes impeded |
| Local (usually postsurgical) pericardial adhesions |
Local cardiac compression by loculated fluid |
| Pulmonary vein and left ventricular diastolic pressures
and left ventricular stiffness markedly exceed those of the
right ventricle* |
Reduced effects of respiration on right-heart filling |
| Right ventricular hypertrophy without pulmonary hypertension |
Causes right-sided resistance to the effects of breathing |
| Severe aortic regurgitation, with or without severe left
ventricular dysfunction |
Produces sufficient regurgitant flow to damp down respiratory
fluctuations |
| Atrial septal defects |
increased inspiratory venous return balanced by shunting
to the left atrium |
| Some cases of low-pressure tamponade |
Makes marked respiratory changes in blood pressure diagnostically
insignifican |
| |
|
* In patients with marked left ventricular hypertrophy
or severe left-sided heart failure, pericardial pressure effectively
equilibrates only with right heart pressures, a form of right
ventricular tamponade, with the much less compliant left ventricle
resisting phasically changing pericardial pressure. Under these
conditions, respiratory changes cannot alternately favor right-
and left-sided filling.
LABORATORY INVESTIGATIONS
Cardiac catheterization will show equilibration
of average diastolic pressure and characteristic respiratory reciprocation
of cardiac pressures: an inspiratory increase on the right and
a concomitant decrease on the left-the proximate cause of pul-sus
paradoxus. Except in low-pressure tamponade, diastolic pressures
throughout the heart are usually 15 to 30 mm Hg. These are similar
to pressures present in heart failure, but for unknown reasons,
tamponade does not cause alveolar pulmonary edema.Although any
type of large silhouette in a patient with clear lung fields should
suggest the presence of pericardial eeffusion,chest films may
not be helpful initially,since at least 200ml of fluid must accumulate
before the cardiac silhouette is affected.In the lateral film,definite
pericardial-fat lines are uncommon but are highly specific for
large effusions.
An electrocardiogram may show signs of peri-carditis, but the only quasispecific sign of tamponade is electrical alternation, which may affect any or all electrocardiographic waves or only the QRS. If the QRS complex is affected, every other QRS complex is of smaller voltage, often with reversed polarity. Combined P and QRS alternation is virtually specific for tamponade. In rare cases, very large effusions, even without tamponade, cause QRS alternation. Echocardiography reveals its mechanism: swinging of the heart (Fig. 2). The volume of most nonhemorrhagic effusion that cause tamponade is moderate to large (300 to 600 ml).
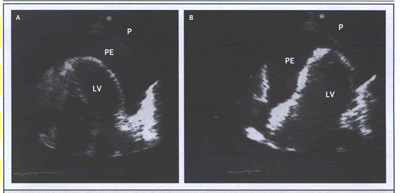
cardTamp-Fig. 2. Swinging of the Heart with a
Large Pericardial Effusion (PE), Causing Electrical Alternation
and Consequent Tamponade.
(click to see video
of Swinging of the Heart)
Apical four-chamber two-dimensional echocardiograms show the extremes of oscillation and the resultant effect on the QRS complex. In Panel A, the heart swings to the right, and lead I I shows a small QRS complex. In Panel B, the heart swings to the left, and the QRS complex is larger. P denotes pericardium, and LV left ventricle.
Doppler echocardiography is the principal tool for diagnosing pericardial effusion and cardiac tamponade. Computed tomography (CT) and magnetic resonance imaging are often less readily available and are generally unneeded unless Doppler echocardiography is not feasible. In the absence of myocardial disease or injury, echocardiography demonstrates the usually circumferential fluid layer and compressed chambers with high ventricular ejection fractions. Doppler study discloses marked respiratory variations in transvalvular flows. One mechanism of pulsus paradoxus is visible: on inspiration, both the ventricular and atrial septa move sharply leftward, reversing on expiration; in other words, each side of the heart fills at the expense of the other, owing to the fixed intrapericardial volume. The inferior vena cava is dilated, with little or no change on respiration.
Among echocardiographic signs, the most characteristic, although
they are not entirely specific, are chamber collapses, which are
nearly always of the right atrium and ventricle. During early
diastole, the right ventricular free wall invaginates, and at
end diastole, the right atrial wall invaginates. Right ventricular
collapse is a less sensitive but more specific finding for tamponade,
whereas right atrial collapse is more specific if inward movement
lasts for at least 30 percent of the cardiac cycle. Right atrial
collapse may be seen in patients with hypovolemia who do not have
tamponade. In about 25 percent of patients, the left atrium also
collapses, and this finding is highly specific for tamponade.
Left ventricular collapse usually occurs under special conditions
such as localized postsurgical tamponade. These wall changes occur
when respective chamber pressures temporarily fall below the peri
cardial pressure.but are highly specific for large effusions.
Among echocardiographic signs, the most characteristic, although
they are not entirely specific, are chamber collapses, which are
nearly always of the right atrium and ventricle. During early
diastole, the right ventricular free wall invaginates, and at
end diastole, the right atrial wall invaginates. Right ventricular
collapse is a less sensitive but more specific finding for tamponade,
whereas right atrial collapse is more specific if inward movement
lasts for at least 30 percent of the cardiac cycle. Right atrial
collapse may be seen in patients with hypovolemia who do not have
tamponade. In about 25 percent of patients, the left atrium also
collapses, and this finding is highly specific for tamponade.
Left ventricular collapse usually occurs under special conditions
such as localized postsurgical tamponade. These wall changes occur
when respective chamber pressures temporarily fall below the peri
cardial pressure.
VARIANT FORMS OF CARDIAC TAMPONADE
Low-pressure tamponade occurs at diastolic pressures
of 6 to 12 mm Hg and is virtually confined to patients with hypovolemia
and severe systemic diseases, hemorrhage, or cancer, or in patients
with hypovolemia after diuresis. Patients are weak and generally
normotensive, with dyspnea on exertion and no diagnostic pulsus
paradoxus, but with characteristic respiratory fluctuations in
transvalvular diastolic Doppler flows. The low-pressure effusion
equilibrates only with right-sided diastolic pressures and does
so at first only during inspiration ("inspiratory tracking").
A fluid challenge with a liter of warm saline can evoke tamponade
dynamics.
Hypertensive cardiac tamponade with all the classic features of
tamponade, occurs at high and very high arterial blood pressures
(even over 200 mm Hg) and is ascribed to excessive betaadrenergic
drive. Affected patients typically have had antecedent hypertension.
Regional cardiac tamponade occurs when
any cardiac zone is compressed by loculated effusions, which are
usually accompanied by localized pericardial adhesions, especially
after cardiac surgery. Sometimes the typical hemodynamic abnormalities
are found only in the compressed chambers or zones. However, loculation
can also produce classic tamponade, presumably by tightening the
uninvolved pericardium; for example, loculated effusions after
cardiac surgery may include hematomas over the right atrium and
atrioventricular groove. Localized right atrial tamponade may
also cause right-to-left shunting through a patent foramen ovale
or an atrial septal defect.
After right ventricular infarction, loculated effusion can cause
selective right-heart tamponade in which right atrial pressure
is higher than left atrial pressure. The absence of pulsus paradoxus
(Table 1) makes this form difficult to recognize. Effusive-constrictive
pericarditis is characterized by mixed clinical, imaging, and
hemodynamic signs, because a constrictive epicarditis underlies
the pericardial effusion. In some patients with scarred, rigid
parietal and visceral pericardium, tamponade can occur with relatively
little accumulation of fluid. Effusive-constrictive pericarditis
is revealed in these patients when drainage of pericardial fluid
does not cause intracardiac pressures to return to
normal.
SPECIAL PROBLEMS
Postoperative tamponade , which is more frequent
after valve surgery than after coronary-artery bypass surgery
and is more frequent with postoperative anticoagulant therapy,
is due to trauma-induced pericardial effusion and bleeding. Since
some degree of pericarditis occurs after every cardiac operation,
and most patients have a small, seemingly benign effusion postoperatively,
it is not surprising that tamponade eventually occurs in some.
Postoperative myocardial stiffness, variable fluid-electrolyte
abnormalities, and hemorrhage tend to preclude the appearance
of classic signs such as pulsus paradoxus (Table 1); thus, when
tamponade is suspected postoperatively, prompt imaging - particularly
Doppler echocardiography-is necessary. Late tamponade, occurring
more than five days postoperatively, must be suspected in any
patient in whom hypotension develops. Primary care physicians
may not be familiar with tamponade, and if it occurs very late
(two weeks or more) after surgery, they may not suspect it. Some
episodes of late hemorrhage may be delayed because the rates of
bleeding are relatively slow and intrapericardial clotting complicates
diagnosis and management.
MANAGEMENT OF ACUTE CARDIAC TAMPONADE
The treatment of cardiac tamponade is drainage of the pericardial
contents, preferably by needle paracentesis (Fig. 3) with the
use of echocardiographic or another type of imaging, such as fluoroscopy
or CT. The needle tip is evident on imaging, and imaging can thus
safely be used to identify the optimal point at which to penetrate
the pericardium.Drainage may be performed in the catheterization
laboratory when the diagnosis is uncertain or effusive constrictive
pericarditis is possible. However, sudden circulatory collapse
warrants the use of pericardiocentesis without imaging, since
further decompensation may occur without warning. If the heart
cannot be reached by a needle or catheter, surgical drainage is
required, usually through a subcostal incision. Surgical drainage
is desirable in patients with intrapericardial bleeding and in
those with clotted hemopericardium or thoracic conditions that
make needle drainage difficult or ineffective. Subcritical uremic
tamponade often responds to intensified renal dialysis, but if
this approach is unsuccessful, drainage is required.
Recurrences, especially in patients with malignant
tamponade, may require balloon pericardiotomy through the use
of special catheters that create "windows" between the
pericardium and the absorbing surface of the pleura or peritoneum.
Death in patients with tamponade is usually heralded by pulseless
electrical activity: the electrocardiogram continues to register
complexes in the absence of blood flow or pressure.
Medical treatment of acute cardiac tamponade, including inotropic
support with or without vasodilators, is relatively controversial
and is aimed at supporting compensatory mechanisms to reduce the
elevated vascular resistance. Thus, dobutamine, administered to
reverse the hypotension, is theoretically ideal. During tamponade,
however,endogenous inotropic stimulation of the heart is often
already maximal.
The approach to medical therapy has been based on studies in animals.
However, these results are the subject of controversy, since in
short-term surgical experiments in anesthetized animals, the presence
of myocardial depression causes almost any measure to improve
function.
Studies in intact, unanesthetized animals with
indwelling instruments and euvolemia have yielded different results
that have cast doubt on the value of various approaches, especially
volume infusion. Indeed, increasing the volume may help only in
patients with hypovolemia, since in patients with normovolemia
and hypervolemia, volume infusion may increase intracardiac pressures
as well as heart size, which in turn increases pericardial pressure,
further reducing or eliminating the low transmural myocardial
pressures supporting the circulation. Moreover, intravenous administration
of resuscitative fluid can precipitate tamponade.
An opioid mechanism contributes to the hypotension of cardiac
tamponade; experiments in animals show that naloxone counteracts
the hypotension, but this approach has not been used clinically.
Mechanical ventilation with positive airway pressure
should be avoided in patients with tamponade, because this further
decreases cardiac output. In patients with cardiac arrest and
a large amount of pericardial fluid, external cardiac compression
has little or no value, because there is little room for additional
filling and because even if systolic pressure rises,diastolic
pressure falls and, in doing so, reduces coronary perfusion pressure.
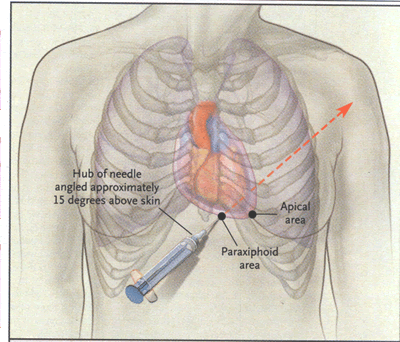
cardTamp-Fig. 3. Most Common Sites of Blind and
Image-Guided Insertion of the Needle for Pericardiocentesis.
In the paraxiphoid approach, the needle should be aimed toward
the left shoulder. In the apical approach, the needle is aimed
internally.
PERICARDIOCENTESIS
Needle drainage of pericardial fluid, whether
or not it is done on an emergency basis (e.g., in a patient in
rapidly worsening hemodynamic condition), requires the clinician
to select a point on the patient's chest or epigastrium to insert
the needle. This is best done with imaging, as already discussed,
to determine which anterior landmarks, usually paraxiphoid or
apical, are closest to the fluid. The paraxiphoid approach is
also most often used for pericardiocentesis that is performed
without imaging. Common points of access are illustrated in Figure
3. The needle is usually inserted between the xiphoid process
and the left costal margin; in patients with tough skin, a small
nick may be made first with a scalpel. The needle is inserted
at a 15-degree angle to bypass the costal margin, and then its
hub is depressed so that the point is aimed toward the left shoulder.
The needle is then advanced slowly, until the pericardium is pierced
and fluid is aspirated. Electrocardiography should not be used
to monitor the patient's condition, since attaching an electrode
to the needle may provide misleading results. The use of a 16-gauge
to 18-gauge polytetrafluoroethylenesheathed needle facilitates
the process, since its steel core can be withdrawn once the pericardium
has been breached, leaving only the sheath in the pericardial
space. For prolonged drainage, a guide wire passed through the
sheath will facilitate the introduction of a pigtail angiographic
catheter. Thereafter, patients should be followed with the use
of Doppler echocardiography to ensure that the pericardial space
has been adequately drained and to avert a recurrence. When the
amount of fluid drained is less than 50 ml a day, the catheter
may be withdrawn; the patient should continue to be observed.
David H. Spodick, M.D., D.Sc.,N ENGL J MED 349;7:684-90
,AUGUST 14, 2003
|