This condition consist of the following
anomalies:
1) a "parachute " deformity of
the mitral valve,
2) supravalvular ring of the left atrium,
3) subaortic stenosis, and
4) aortic coarctation (figures
23a).
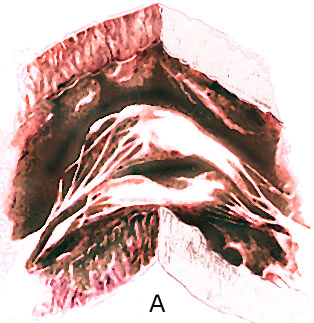
Figure 44g-1
The " parachute mitral valve"
has the usual two mitral valvular leaflets and commissures,
but the chordae, instead of diverging to insert into two papillary
muscle, converge into one major papillary muscle (figure 44g-1
and figure 44g-2).
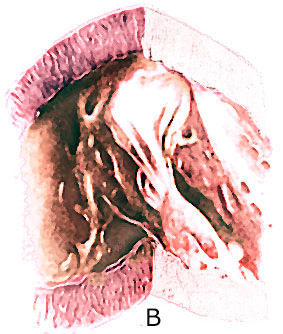
Figure 44g-2
The analogy to a parachute is suggested
by the shape of the deformed valve. The mitral leaflets resemble
the canopy of a parachute, the chordae, its shrouds or strings,
and the papillary muscle, the harness. The chordae are often
short and thick; this, coupled with theirconvergent papillary
insertion,allows little mobility of the leaflets. The effect
creates a stenotic mitral valve since the leaflets are held
in close apposition. The only effective communication between
the left atrium and the left ventricle is through the interchordal
spaces. In aggregate these spaces did not allow free egress
of blood from the left atrium.
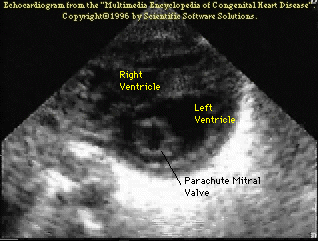
The following (click
here for video) and picture shows an example of a parachute
mitral valve.
SUPRAVALVULAR RING OF THE LEFT ATRIUM
This entity is a circumferential ridge of
connective tissue that arises at the base of the atrial surface
of the mitral leaflets and protrudes into the inlet of the
mitral valve. In the fully developed deformity it acts as
a stenosing, perforated diaphragm. In some cases it protrudes
only slightly and causes no obstruction to the egress of blood
from the left atrium.
Background:
Supravalvar mitral ring is a rare congenital
heart defect, of surgical importance, characterized by an
abnormal ridge of connective tissue on the atrial side of
the mitral valve. Often circumferential in shape, the supravalvar
ring may encroach on the orifice of the mitral valve and may
adhere to the mitral valve leaflets and restrict their movements.
While a supravalvar mitral ring may allow normal hemodynamic
flow from the left atrium to the left ventricle (LV), it often
causes significant obstruction to mitral valve inflow.
While it can occur as an isolated defect,
in nearly 90% of patients supravalvar mitral ring is found
in combination with other congenital heart defects. Awareness
of anatomic variations in patients with supravalvar mitral
ring and preoperative recognition of the lesion are important.
Pathophysiology:
Supravalvar mitral ring is a circumferential ridge or membrane
arising from the left atrial wall overlying the mitral valve
and frequently attached to the mitral valve annulus. Variable
in thickness and extent, it ranges from a thin membrane to
a thick discrete fibrous ridge. The membranous variety may
be difficult to detect, since the membrane often adheres to
the anterior mitral valve leaflet while remaining just proximal
to the posterior mitral leaflet. Adhesion to the valve may
impair opening movement of the leaflets, and this may be the
main mechanism of mitral valve inflow obstruction in some
patients.
In others, the ring may be large enough to protrude into
the mitral valve inflow and cause obstruction. The supramitral
ring also may be incomplete and eccentric, allowing unobstructed
flow through the mitral valve.
Supravalvar mitral ring rarely occurs as an isolated defect,
and other congenital heart defects coexist in most (90%) patients.
The mitral valve itself often is abnormal and stenotic at
the valvar or subvalvar level with fusion of leaflets, small
valve orifice, and abnormal papillary muscles being common
abnormalities.
Shone syndrome describes a combination of 4 congenital heart
defects: supravalvar mitral ring, parachute mitral valve,
subvalvar aortic stenosis, and aortic coarctation.
Other common associated lesions in patients with supravalvar
mitral ring are ventricular septal defect (VSD), patent ductus
arteriosus (PDA), atrioventricular canal defect, and tetralogy
of Fallot.
Less commonly associated defects include atrial septal defect,
left superior vena cava, and Wolff-Parkinson-White syndrome.
Lesions such as transposition of the great arteries and double
outlet right ventricle uncommonly are complicated by the presence
of a supravalvar mitral ring.
Obstruction to mitral inflow results from a reduction in
mitral valve orifice area. When significant, a diastolic pressure
difference occurs between the left atrium and LV. Left atrial
and pulmonary venous pressures increase, leading to exudation
of fluid into the pulmonary interstitium, which causes increased
lung stiffness. Breathlessness and tachypnea are secondary
to the interstitial edema and diminished pulmonary compliance.
In severe cases, frank pulmonary edema can occur.
An associated atrial septal defect may decompress the left
atrium, thereby reducing or masking severity of the mitral
valve obstruction.
Associated lesions, such as VSD or PDA, which increase LV
output, will exacerbate the manifestations of mitral inflow
obstruction. Conversely, supravalvar mitral ring may be difficult
to detect in the presence of conditions with diminished pulmonary
blood flow, such as tetralogy of Fallot.
Persistently elevated pulmonary venous hypertension leads
to pulmonary arterial hypertension, a rise in pulmonary vascular
resistance, and eventually, failure of the right ventricle.
Tricuspid regurgitation is a common accompaniment of right
heart failure from pulmonary hypertension.
Frequency:
Internationally: No data are available on incidence of supravalvar
mitral ring. In most patients, the supravalvar mitral ring
is detected during investigation for other congenital heart
disease (CHD).
Race: No specific race predilection exists.
Sex: No specific sex predilection exists.
Age: No specific age predilection exists.
History: Supravalvar mitral ring can be diagnosed in one
of the following ways:
Supravalvar mitral ring most commonly is diagnosed as an
associated finding in other CHD.
Supravalvar mitral ring occasionally may be found as the
cause of congenital mitral stenosis (MS) in symptomatic children
with dyspnea or pulmonary hypertension. The severity of symptoms
depends upon the level of left atrial and pulmonary venous
hypertension.
Most patients become symptomatic by age 2 years.
Rarely, this condition may be detected as an incidental
finding in asymptomatic patients undergoing echocardiography
for some unrelated reason.
Symptoms of supravalvar mitral ring with MS include one
or more of the following:
1. Dyspnea, nocturnal cough, and tachypnea from pulmonary
venous congestion and increased lung stiffness
2. Frequent respiratory infections and wheezing from pulmonary
congestion, increased fluid exudation, and airway narrowing
3. Poor feeding, failure to thrive, fatigue, and sweating
from heart failure and reduced cardiac output
4. Occasionally acute pulmonary edema or generalized edema
5. Hemoptysis and syncope in older patients
Physical: Physical signs in supravalvar mitral ring usually
relate either to the associated CHD or to pulmonary arterial
hypertension. Children with significant mitral obstruction
frequently are quite sick, with tachypnea and respiratory
distress. Diminished cardiac output and poor perfusion lead
to a low volume pulse and peripheral cyanosis. Systemic venous
pressure may be elevated with the development of congestive
heart failure (CHF). A prominent parasternal heave indicates
right ventricular hypertrophy from pulmonary hypertension.
The pulmonary component of the second heart sound is accentuated,
Yet, unlike acquired mitral valvar stenosis, an opening snap
of the mitral valve is not heard in supravalvar mitral ring.
An apical middiastolic murmur of MS may be audible at the
apex, especially in the left lateral decubitus, and it may
exhibit presystolic accentuation. The murmur is very prominent
when supravalvar mitral ring is associated with VSD or PDA,
causing a large mitral inflow.
Patients with chronic mitral obstruction develop signs of
tricuspid regurgitation and CHF, such as hepatomegaly, engorged
neck veins, large expansile CV waves in the jugular venous
pulse, and a systolic murmur that accentuates in inspiration
at the lower left sternal border.
DIFFERENTIALS
Cor Triatriatum
Mitral Valve, Double Orifice
Other Problems to be Considered:
Pulmonary hypertension, congenital heart disease
Lab Studies:
No specific laboratory blood tests are required for diagnosis.
Imaging Studies:
1. Imaging studies are essential to define the anatomy of
the ring and mitral valve, to assess the severity of obstruction,
and to identify any associated defect before undertaking surgical
treatment.
2. Chest x-ray
a. Left atrial enlargement, the most common abnormality on
chest x-ray in patients with mitral obstruction, is diagnosed
by the findings of straightening of the left cardiac border
(mitralization), widening of the tracheal carina, and elevation
of the left bronchus. In older children, the enlarged left
atrium may be seen as a double density near the right cardiac
border.
b. The left atrium tends to enlarge in a posterior direction.
A barium-swallow study of the esophagus in lateral projection
shows a rounded indentation of the anterior wall.
c. Prominent upper lobe pulmonary veins, increased interstitial
markings, and Kerley lines indicate pulmonary venous hypertension.
In severe cases, alveolar edema produces a hazy appearance
in the hilar regions of both lung fields.
d. The pulmonary trunk and its branches become dilated with
the rise in pulmonary arterial pressure. Cardiac contour reflects
right ventricular hypertrophy.
3. Echocardiography
a. Two-dimensional echocardiogram with Doppler is the most
important tool for the diagnosis and detailed assessment of
patients with supravalvar mitral ring. It identifies the lesion
and quantifies severity of the obstruction.
b. Perform a systematic and diligent scan of the mitral valve
and left atrium, using multiple transthoracic views and paying
particular attention to evaluate all components of the mitral
valve apparatus. Use parasternal, apical, and subcostal views
to visualize the mitral inflow region.
c. Using this technique allows the physician to view the
supravalvar mitral ring and define its exact position, size,
and extent, and to assess the relation of the ring to the
mitral valve leaflets.
d. Occasionally, a thin membrane may so closely adhere to
the valve leaflets that it is difficult to demonstrate by
2-dimensional echo. With an adherent membrane, the movements
of mitral valve leaflets may be impaired, characterized by
diminished excursions and a flattened E-F slope on motion
mode (M-mode) echo of the mitral valve.
e. Inspect mitral valve chordae and papillary muscles for
any associated abnormality. Exclude other associated defects,
particularly subaortic stenosis, VSD, and coarctation of the
aorta.
f. The pulmonary artery, right ventricle, and right atrium
enlarge with the development of pulmonary arterial hypertension.
g. Use M-mode echocardiography of the pulmonary valve, which
often shows such signs of pulmonary hypertension as an abbreviated
A wave, midsystolic closure, and systolic flutter of pulmonary
leaflets.
4. Doppler echocardiography
a. Doppler interrogation and color-flow mapping reveal the
pattern of flow through the mitral valve, diagnose the presence
and severity of obstruction, and demonstrate additional areas
of abnormal flow in valvar or subvalvar mitral regions. The
characteristic finding is turbulent flow with increased velocity
across the supravalvar mitral ring into the mitral valve.
b. Quantify the severity of mitral obstruction by measuring
the mean velocity of diastolic flow through the mitral valve.
The mean diastolic velocity as well as the pressure half-time
(time taken for the peak diastolic velocity to fall to half
its initial value) correlate well with the severity of obstruction.
c. Measure the peak velocity of the tricuspid regurgitant
jet in the right atrium for an estimate of systolic right
ventricular pressure.
Other Tests:
5. Transesophageal echocardiography
a. Transesophageal echocardiography generally is not necessary
to assess supravalvar mitral ring with obstruction in children,
as adequate information can be obtained from transthoracic
windows.
b. In older patients, heavily built individuals, and in patients
with emphysematous chests, transesophageal study can provide
additional, clear views to inspect all components of the supravalvar
mitral ring and mitral valve.
c. Thrombi in the left atrium may be detected.
d. Intraoperative transesophageal echo is useful for patients
of all ages to assess adequacy of repair in the operating
room.
5. Electrocardiogram
a. The electrocardiogram in isolated supravalvar mitral ring
demonstrates left atrial enlargement, right ventricular hypertrophy,
and right atrial enlargement in proportion to the degree of
obstruction.
b. Presence of additional defects will influence the electrocardiogram
accordingly.
Procedures:
1. Cardiac catheterization
a. Cardiac catheterization is not necessary if echo provides
all anatomic and hemodynamic data in patients with supravalvar
mitral ring; however, it can provide additional information
on the severity of mitral obstruction, especially in the presence
of other associated CHD.
b. Proximal left atrial pressure and pulmonary venous pressure
both are elevated. A pressure difference can be demonstrated
in diastole between the left atrium and LV. Since entry into
the left atrium may be difficult and require transseptal puncture,
pressure recorded in the pulmonary artery wedge position usually
is a reliable indicator of left atrial pressure.
c. Pulmonary artery pressure is elevated in chronic mitral
obstruction. Associated shunts and other obstructive lesions
also are identified and quantified at cardiac catheterization.
2. Cardiac angiography
a. With the availability of high-quality 2-dimensional and
Doppler echocardiography, cardiac angiography has a limited
role in the assessment of patients with supravalvar mitral
ring. Echocardiography is superior to angiography in defining
the anatomic and functional abnormality.
b. Left atrial angiogram in the caudally angulated right
anterior oblique view and 4-chamber view may demonstrate the
supravalvar mitral ring. A closely adherent ring may, however,
be difficult to visualize and differentiate from valvar mitral
stenosis, since the left atrium and appendage are enlarged
and clearance of contrast from the left atrium into the LV
is delayed.
c. An LV angiogram provides additional anatomic information
about the mitral valve, ventricular septum, left ventricular
outflow tract, and aortic arch.
TREATMENT
Medical Care: Evaluate patients with supravalvar mitral ring
on an outpatient basis. Admit patients to the hospital for
cardiac catheterization, treatment of severe heart failure
or pulmonary edema, and for surgical treatment.
1 Goals of medical treatment
a. To relieve symptoms caused by pulmonary venous congestion
and CHF
b. To stabilize the patientís condition before undertaking
detailed assessment and surgical repair
c. To serve as an adjunct to surgical repair in the postoperative
period
D. Control of heart failure by medical therapy may be the
preferred option in small infants. Controlling CHF may permit
deferral of surgery temporarily.
Surgical Care:
1. Goals of surgical therapy
a. Perform surgical repair in all symptomatic patients with
supravalvar mitral stenosis to relieve the obstruction.
b. Perform an early operation for supravalvar mitral ring
in the presence of severe heart failure, pulmonary edema,
or pulmonary arterial hypertension.
c. Adjust the type of operation depending on the anatomy
of the supravalvar ring and mitral valve apparatus and any
associated congenital heart defect. Make every effort to define
the anatomy in detail before undertaking surgery. In many
patients, the supravalvar ring can be excised completely while
any associated mitral valve abnormality is repaired simultaneously.
If the supravalvar ring is densely adherent to the mitral
valve leaflet or the mitral valve apparatus is grossly abnormal,
replacement of the mitral valve may be necessary.
d. Selected cases of supravalvar ring with mitral stenosis
may be amenable to balloon dilatation, but results are less
successful than with operation.
2. Presence of a normal underlying mitral valve is associated
with a better surgical outcome than with abnormal valve tissue.
a. In patients who require resection at an early age the
prognosis is poor. Mortality is high, with risk of recurrent
supravalvar mitral stenosis in survivors, probably because
of continuing turbulence across the small LV inflow tract.
Consultations: Consult a cardiologist and cardiothoracic
surgeon.
Diet:
1. No special diet is required in asymptomatic patients with
supravalvar mitral ring.
2. Advise patients to avoid excess intake of salt or to reduce
salt intake in the presence of heart failure. Use salt restriction
cautiously in infants.
3. Restrict fluid intake to approximately 60-80 mL/kg/d in
infants with CHF.
Activity: Advise patients with pulmonary venous congestion
or CHF to avoid strenuous exertion. Asymptomatic children
without pulmonary hypertension may participate in normal activities.
MEDICATION
Caption: Picture 1. Mitral stenosis, supravalvular ring.
Seen here is a 2-dimensional echocardiogram in parasternal
long-axis view showing a supravalvar mitral ring (small arrows)
close to and adherent to the mitral valve leaflet (large arrow).
The ring and the restricted opening of the mitral valve cause
mitral obstruction. A large ventricular septal defect also
is present. LA = left atrium, LV = left ventricle, AO = aorta,
RV = right ventricle
Caption: Picture 2. Mitral stenosis, supravalvular ring.
Seen here is a 2-dimensional echocardiogram with color flow
imaging in the parasternal long-axis view showing turbulent
flow (arrow) in diastole from left atrium (LA) to left ventricle
(LV), caused by an obstructive supravalvar mitral ring. RV
= right ventricle.
Caption: Picture 3. Mitral stenosis, supravalvular ring.
This 2-dimensional echocardiogram in the apical view shows
the supravalvar mitral ring (small arrows) adherent to the
mitral valve leaflet (large arrow). LA = left atrium, LV =
left ventricle, RA = right atrium, RV = right ventricle.
Caption: Picture 4. Mitral stenosis, supravalvular ring.
This image is a 2-dimensional echocardiogram with color flow
imaging in apical view showing turbulent flow (arrow) in diastole
from left atrium (LA) to left ventricle (LV), caused by an
obstructive supravalvar mitral ring. RA = right atrium, RV
= right ventricle
Caption: Picture 5. Mitral stenosis, supravalvular ring.
Simultaneous recording of pressures in the pulmonary artery
wedge position (PAW) and the left ventricle (LV) shows a large
gradient in diastole across the mitral valve. The PAW pressure
is markedly elevated.
Picture 6. Mitral stenosis, supravalvular ring. Shown here
is an M-mode echocardiogram of the mitral valve in a patient
with supravalvar mitral ring causing obstruction. The mitral
valve leaflets show diminished excursion and a markedly reduced
E-F slope in diastole. RV = right ventricle, LV = left ventricle,
MV = mitral valve.
SUBAORTIC STENOSIS
Two types of subaortic stenosis occur---the
muscular and the membranous. The muscular type is characterized
by localized protusion of hypertrophied ventricular septal
tissue into the left ventricular outflow tract. The membraneous
type is characterized by circumferential endocardial thickening
in the left ventricular outflow tract. In some cases the two
types co-exist.
COARCTATION of the AORTA
Coarctation of the aorta (figure
23a) may coexist as well.
Shoke,J.D.,and
others,The Developmental Complex of "Parachute Mitral
Valve",Supravalvular Ring of Left Atrium,Subaortic Stenosis,And
Coarctation of Aorta,American Journal Cardiology,1963;11:714-725.
Brickner,M.E.
and others,Congenital Heart Disease inAdults, N.Engl.J.Med.,
Vol.342.N.4, Jan.27,2000