This
term refers to an abnormality in the aorta, be it in the thoracic
or abdominal portion. The abnormality is a marked dilatation
of a particular portion (focal or diffuse, saccular or fusiform)
of the aorta (see figure
51b). The dilatation is due to a number of factors:
1)
degeneration of the walls of the aorta, including the loss of
elastic tissue and smooth muscle cells in the medial portion
of the wall, with replacement by scar tissue (collagen) and
a ground substance;
2)
atherosclerosis (the major cause, see figure
70), a process of lipid deposition into the walls of the
aorta, followed by scarring and ulcerations covered with thrombi
(blood clots), which can break away and go downstream as emboli
to clog up smaller vessels;
3)
infections, including syphilis (see figure
48d), fungi, and bacteria (see figures:
48c, 48e,
48f);
4)
associated tissue changes of inflammation as in ankylosing spondylitis;
5)
congenital anomalies
The Loeys–Dietz syndrome is a recently described
autosomal dominant aortic-aneurysm syndrome with widespread
systemic involvement. The disease is characterized by the triad
of arterial tortuosity and aneurysms, hypertelorism, and bifid
uvula or cleft palate and is caused by heterozygous mutations
in the genes encoding trans-forming growth factor /3 receptors
1 and 2 (TGFBR1 and TGFBR2, respectively).
METHODS
The clinical and molecular characterization
of 52 affected families were performed. Forty probands presented
with typical manifestations of the Loeys–Dietz syndrome. In
view of the phenotypic overlap between this syndrome and vascular
Ehlers–Danlos syndrome, an additional cohort of 40 patients
who had vascular Ehlers–Danlos syndrome without the characteristic
type III collagen abnormalities or the craniofacial features
of the Loeys–Dietz syndrome were studied.
RESULTS
A mutation in TGFBR1 or TGFBR2 was found
in all probands with typical Loeys–Dietz syndrome (type I) and
in 12 probands presenting with vascular Ehlers–Danlos syndrome
(Loeys–Dietz syndrome type II). The natural history of both
types was characterized by aggressive arterial aneurysms (mean
age at death, 26.0 years) and a high incidence of pregnancy-related
complications (in 6 of 12 women). Patients with Loeys–Dietz
syndrome type I, as compared with those with type II, underwent
cardiovascular surgery earlier (mean age, 16.9 years vs. 26.9
years) and died earlier (22.6 years vs. 31.8 years). There were
59 vascular surgeries in the cohort, with one death during the
procedure. This low rate of intraoperative mortality distinguishes
the Loeys–Dietz syndrome from vascular Ehlers–Danlos syndrome.
CONCLUSIONS
Mutations in either TGFBR1 or TGFBR2 predispose
patients to aggressive and wide-spread vascular disease. The
severity of the clinical presentation is predictive of the outcome.
Genotyping of patients presenting with symptoms like those of
vascular Ehlers–Danlos syndrome may be used to guide therapy,
including the use and timing of prophylactic vascular surgery.
FIGURE 2
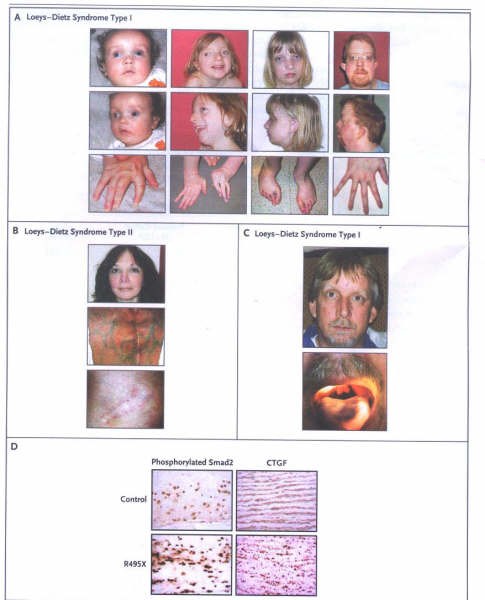
Characteristics of the Loeys–Dietz
Syndrome.
Panel A shows typical facial characteristics
of patients with Loeys–Dietz syndrome type I at different ages:
blue sclerae, hypertelorism, proptosis, malar flattening, retrognathia,
camptodactyly, and arachnodactyly. Panel B shows the facial
characteristics of a patient with Loeys–Dietz syndrome type
II. The translucency of the skin is evident, with visible veins
and distended scars. Panel C shows a patient who had type I
with a nonsense mutation (R495X) in TGFBR2, hypertelorism, and
bifid uvula. Panel D shows the results of immunostaining of
aortic tissue from a patient who was heterozygous for the R495X
mutation, revealing increased nuclear accumulation of phosphorylated
Smad2 and levels of expression of connective-tissue growth factor
(CTGF), both indicative of increased TGF-B signaling, as compared
with an age-matched control.
The National Istitutes of Health is sponsoring
a clinical trial that will compare losartan (angiotensin II
type1-receptor antagonist with beta -blocker therapy in children
and young adults with Marfan's syndrome and aortic aneuysm.
From The N ENGL J MED 355;8(August24,
2006), Pages 788-798.
6)
hypertension.
The
opening of the aneurysm usually contains a laminated thrombus
that may or may not completely fill the aneurysm.
Thoracic aortic aneurysms (see figures
above: 50, 51a, 51b, 51c, 51d, 51e, 51f, 51g) have a five year
mortality, which approaches 75%. One third to one half of these
deaths result from rupture of the aneurysm (see figures
51d and 51e).
Surgical repair constitutes the only
effective treatment for thoracic aneurysms. It is urgently indicated
in patients with a large aneurysm (6 cm or larger), especially
if symptoms suggest expansion or compression of an adjacent
structure.
Cardiac failure from aortic regurgitation
or aortocameral fistula may also necessitate early operative
treatment.
Resection is less urgent in small, asymptomatic
aneurysms.
Consideration of the severity of associated
diseases is also important in selection of patients for surgery.
Surgical treatment consists in replacing
the resected aneurysmal segment with a Dacron graft attached
to relatively normal aorta proximally and distally.
Specific surgical procedures vary with
the site of the aneurysm and the need for maintaining circulation
to distal parts of the body during the necessary period of aortic
occlusion (see figure
51c).
Aneurysms of the abdominal aorta are common;
about 114,000 new cases are diagnosed each year. An abdominal
aortic aneurysm, which is usually located in the infrarenal
portion of the vessel, is defined as an enlargement that exceeds
the normal diameter by 50% or more. Conventially, an abdominal
aortic aneurysm measures more than 3 cm in diameter ( see
figure 171-1 and -2 ). The primary complication is rupture,
which leads to 15,000 deaths per year in the US and makes abdominal
aortic rupture the 13th leading cause of death in this country.
Prophylactic repair is therefore recommended for aneurysms that
are more than 5 cm in diameter.
Endovascular repair of abdominal aneurysms
with stent grafts is a new image-guided, catheter-based approach
that provides a valuable alternative to standard open surgical
repair. Radiologic imaging plays an essential role in preprocedure
evaluation, the procedure itself, and patient followup. The
ultimate goal remains the same - Complete exclusion of the aneurysm
sac to prevent rupture ( see figure
171-3 and-4 ).
Stent graft design
is an intraluminal device that consists of a supporting metal
framework and synthetic graft M material that is either self-expanding
or balloon-expandible. Percutaneous delivery is made possible
by compacting the device onto a catheter or compressing it into
a sheath. Stent grafts are available in three basic forms, including
tube, bifurcated, and aorta-unilateral designs.
About 60% of patients
with abdominal aortic aneurysms are eligible for endovascular
stent graft repair.
Morbidity rates have
been reported at 23% for surgery and 12% for endovascular repair.
Hospital stay is reduced by two-thirds, to 3.4 days. Rupture
is rare. One critireon for success is the absence of endoleaks,
which are indicated by persistent opacification of the aneurysm
sac after insertion of the stent graft ( see
figure 173-6 ).
Reference:Montgomery,M.L.,MD
and Sullivan,J.P.,MD,Advances in interventional radiology,Postgraduate
Medicine,Vol.109,No.6,June 2001,Pp97-98.