AORTIC
VALVE
MITRAL
VALVE
PULMONIC
VALVE
TRICUSPID
VALVE
Replacement Heart Valves
By comparison with the revolutionary changes
in many other fields of medicine, the development of artificial
heart valves has progressed rather gradually since its beginning
in 1960. The main problems recognized with the first designs-thromboembolism
in mechanical valves and structural deterioration in tissue
valve were reduced to an acceptable level by 1965-1970, and
have not been substantially reduced further in the ensuing 25
years. However, the past few years has seen a resurgence in
the development cycle, and there are currently many new devices
are under investigation
Background
Heart valves can be classified into
two broad categories according to the origin of their occluding
mechanism: mechanical and biological. After the heart-lung machine
made valve replacement a possibility in 1955, many replacement
devices were attempted. Mechanical and biologic valves of all
kinds were tried, including designs with one, two, three and
four leaflets. The first valve replacements that led to long
term survivors were mechanical cage ball valves used by the
Harken in the aortic position and Starr in the mitral position
both in 1960.
Once the early problems of valve fixation and durability were
solved, the major force that drove mechanical valve development
was the reduction of thromboembolic complications. One favorite
design was the tilting disc valve of which several varieties
exist. The original versions of the bileaflet valve did not
endure, but this design was successfully implemented in 1977
on the basis of the transference of pyrolytic carbon technology
from spacecraft to heart valves. Thus the mechanical valve designs
that have prevailed until today are the ball valve, tilting
disc valve, and the bileaflet valve (figure107).
Current development of mechanical valves is concentrated in
attempting to enhance the bileaflet vave design.
The first biological valves used successfully
were transplants from human cadavers, called homografts or allographs
pioneered by Ross and Barratt-Boyes in 1962. Successful use
of autologous grafts was begun with the pulmonary autograph
in 1967. The goal of these biologic valves was to reduce the
complications associated with thromboembolism and the need for
anticoagulation. Several homologous and heterologous materials
were used to fabricate tissue valves but eventually abandoned.
During the 1960s, a major advance was the use of glutaraldehyde
preservation of porcine valves pioneered by Carpentier and coworkers.
Glutaraldehyde-fixed valves currently in use are aortic porcine
valves and, in resurgence, valves fabricated of bovine pericardium.
Current developments in tissue valve technology include improved
methods of fixation, calcification mitigation treatments, and
stentless designs.
Valve Descriptions
A heart valve functions as a check valve,
opening to permit forward blood flow and closing to prevent
retrograde flow, about 40 million times per year. Heart valve
prostheses consist of an orifice, through which blood flows,
and an occluding mechanism that closes and opens the orifice.
There are two fundamental approaches to valve design: mechanical
prostheses with rigid manufactured occluders, and biological
prostheses also called tissue valves with flexible leaflet occluders
of animal origin. The latter category includes replacement valves
of human origin .
Mechanical Valves
The type of a mechanical valve is designated
by its occluder: a ball, a circular disk or two semi- circular
leaflets (bileaflet). For ball (Fig.107a-upper
left) and disk valves (fig.107b-upper
right), the occluder is guided and retained by structural
members call struts attached to the orifice. The combination
of orifice and struts is referred to as the valve housing. For
bileaflet valves (fig.107c-mid left)
the leaflets are retained and guided by a hinge or pivot mechanism:
projections of the leaflets fit into indentations or sockets
in the housing, which serve to retain the leaflets and define
their limits of travel.
Figure 107 click
to enlarge
Caged Ball Valve
The first clinically successful heart valve
was the Starr-Edwards caged ball valve introduced in 1960. For
5 years the valve underwent several slight design modifications
resulting in the model currently used. The ball is a silicone
rubber polymer impregnated with barium sulfate for radiopacity.
The cobalt- chromium alloy struts are joined at the apex to
form a cage (fig.107a-upper left).
When the ball opens by moving to the end of its cage it creates
a circular primary orifice and a ring shaped secondary orifice
between the ball and the housing. In the aortic position there
is a tertiary orifice between the equator of the ball and the
aorta.
Figure 107f below is copied from the New England Journal of Medicine, May 22, 2008 edition, Image of the Week,
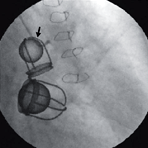
This 67-year-old woman presented with dyspnea and severe tricuspid regurgitation. The mitral and aortic valves from 38 years earlier were functioning normally. Click here for video
Tilting Disk Valve
Tilting disk valves have separate projections
into the orifice, either single arms or closed loops to retain
and guide the disk-shaped occluder. Among the metals use for
the housing are stainless steel and titanium. The disks are
graphite with a coating of pyrolitic carbon.When the disk pivots
to the open position, the primary orifice is separated into
twoareas, called the major and minor orifices. The Bjork-Shiley
valve was the first successful tilting valve. It became available
in 1971 with a carbon-coated disk and both struts (inflow and
outflow) welded to the chromium alloy orifice. The Convexo -
Concave model introduced in 1979, had an integral inflow strut
to eliminate the few inflow strut fractures that had occurred
with previous models. Sorin manufactured a tilting disk valve
patterned after the Shiley valve disk, but with both struts
integral to the housing to avoid the possibility of strut fracture.
It is currently available with a pyrolitic carbon-coated sewing
ring and and entire housing.
The Medtronic Hall valve has a titanium housing
machined from a solid cylinder and a thin carbon coated disk
with flat parallel sides (fig.107b-upper
right) . The disk opens to 75° in the aortic model and 70
degrees in in the mitral. The disc is retained and guided by
an S-shaped guide strut that protrudes through a central hole
in the disk. Four structural elements project perpendicularly
from the annulus into to the orifice: a guide strut and three
pivot struts (one inflow two outflow). The two inflow pivot
struts from near the top (inflow) edge of the orifice toward
each other; the disk seats on the on the flat triangular bottom
surfaces of these struts. The Omniscience valve is a streamlined
elegant looking valve. It has a curved pyrolitic Carbon disk
with no indentations, a one- piece titanium cage, and a seamless
polyester knit sewin ring. The disk opens to 80 degrees and
closes at an angle of 12 degrees to the plane of the orifice.
It has been in use since 1978 but underwent a design change
in 1981 to 82 involving a significant modification of the sewing
ring.
Bileaflet Valves
The currently available bileaflet valves vary
with regard to several design features Although the features
of the valves manufactured by St. Jude, Baxter, Carbomedics,
Soren, ATS, and Medtronic are described here , clinical performance
information is included only for the St. Jude valve, for which
a large amount of long-term information is available. The two
leaflets of a bileaflet valve swing apart during opening, resulting
in three separate flow areas. the bileaflet valve housings are
either solid pyrolytic carbon over a graphite ( titanium in
the Sorin valve) substrate. All except the St. Jude and Sorin
valves have stiffener rings to strengthen the housing, shield
it from needles during implantation, and improve radiographic
visualization (Sorin valve has a titanium substrate, rather
than graphite, which may confer some benefit). All except St.
Jude are rotatable after the plantation. Since its first implant
in 1977, the St. Jude bileaflet valve has been used half a million
times (fig.107c-mid left). Previous
bileaflet valves were unsuccessful; but the results with this
design, with pyrolitic carbon coated housing and leaflets, introduced
a new generation of mechanical prostheses. The housing of the
valve included two rounded tabs, called pivot guards, that project
out from the inflow side. The inside surfaces of these tabs
contain the butterfly shaped indentations that serve to retain
the leaflets. The tabs containing the hinge sockets extend above
the housing, whereas with the other bileaflet valves, the cavities
in the housing containing the pivot recesses are located within
the main body of the housing, approximately at the plane of
the annulus. The leaflets open to 85° and swing through an arc
of 55 to 60°, depending on valve size, from fully closed to
fully open (travel arc).
The TEKNA valve (Baxter Edwards) originally
called Duromedics introduced several modifications of the bileaflet
configuration. The leaflets are curved and translate slightly
in the direction of flow as they open. Unlike other by leaflet
valves, the leaflets seat on a lip or shelf molded into the
housing. This seating design may reduce regurgitation and the
possibility for suture entrapment, but at the expense of an
increase gradient and higher closing impact. In the aortic model,
the leaflets open to 77°, with a travel arc of 62 degrees; these
dimensions are in the mitral, 73° and 58° ,respectively. The
original Duromedicson valve experienced some fractures of leaflets
and housing; it was withdrawn from the market in 1988, reintroduced
in 1990, Carbomedics, the company that made pyrolitic carbon
components, in 1993 received the third marketing approval for
a bileaflet valve in the United States. The carbomedics bileaflet
valve has flat leaflets that open 78 to 80°, with the resultant
travel arc of 53 to 55°; it has a carbon coated blood contacting
surface on the sewing ring.
The Medtronic Parallel bileaflet valve is
unique in that the leaflets open to the maximum possible angle,
90°, to the plane, of the housing with the travel arc of 50°.
Unique design features include an active dual mode pivotl washing
and a housing profile optimized for flow.
Biological Valves
Biological valves include as wide a variety
as do mechanical valves.
1. An autograft
is a valve that has been translocated within the same individual
(e.g., the pulmonary valve in the aortic position).
2. An autologous
tissue valve is a valve that has been fabricated from the patient's
own nonvalvular tissue (e.g. pericardium).
3. A homograft
valvet is one that has been transplanted from a donor of the
same species (a donor's aortic or pulmonary valve into a recipient's
aortic or pulmonary position).
4. A heterograft
is one that has been transplanted from another species; it may
be either an intact valve ( e.g., a porcine aortic valve, as
in fig. 107d-mid right) or a valve
fashioned from heterologous tissue (e.g., bovine pericardium
as in fig. 107e-lower left). The
first successful biological valves were homografts. The homograft
valve is not a homogenous type of valve, but it has appeared
in a range of subtypes according to many variable factors. Sterilization
methods used include chemical (ethylene oxide, beta propiolactone),
rradiation, and antibiotics, with antibiotics being favored
today. Preservation for a short time (months) is accomplished
with nutrient storage of 4°C but cryopreservation, which allows
for indefinite storage, greatly increases the availability of
homografts. Because of supply limitations with homografts, the
most widely used valves are the partially manufactured heterograft
valves. Bioprosthesis is a term Carpentier and Dubost introduced
for a biological tissue that has been treated to render it nonviable.
Glitaraldehyde is used for fixing and preserving prosthetic
heart valves because of three important biological actions:
it sterilizes the tissue, renders it bio acceptable by destroying
antigenicity, and stabilizes the molecular cross- links between
the collagen fibers to enhance durability.
Homograft, Autograft
The homograft valve is considered to be the
preferred substitute for aortic valve replacement, especially
for younger patient. It has excellent hemodynamics, no anticoagulant
requirements, and low (or in some cases zero) thrombogenicity.
The drawback is low availability and a more technical the demanding
operation. The pulmonary autograph procedure consists of an
autotransplant of the pulmonary valve to the aortic position.The
pulmonary valve is then replaced by an aortic or pulmonary homograft.
The pulmonary autograft is perhaps the best aortic valve substitute
for younger patients, as there is potential for growth of the
pulmonary valve in the aortic position ; but this operation
involves a double valve replacement with the attendant early
and late risks.
Autologous Percardial Valve
An innovative Valve concept has recently
has been deloped and is being investigated. This is a new category
of valve: an attempt to combine the reproducibility and the
ease of insertion of the commercial stented heterograft valve
and the benefits of autologous tissue. It is a frame-mounted
autologous pericardial valve, which is assembled from a kit
in the surgical theater. The kit consist of the tools to create
the valve in a matter of minutes: a cookie- cutter- type tool
for obtaining the correctly shaped piece of pericardium, a frame
that snaps together around the tissue, and holder to precisely
align two pieces for assembly.
Porcine Heterograft Valves (Stented)
Most heterograft valves are mounted on rigid
or flexible stents, to which are attached the leaflets and the
sewing ring. Implantation involves fixing the sewing ring into
place in (or above) the patient's annulus. The Hancock standard
porcine valve (Medtronic, Inc.) was the first commercially available
porcine valve. The stent is made up of flexible polypropylene
cylinder with the radiopaque ring of cobalt-chromium alloy added
for rigidity (fig.107d-mid right).
The Hancock modified orifice was designed to overcome the undesirable
hemodynamics caused by the muscular shelf of the porcine right
coronary cusp by replacing that leaflet with one of the other
two leaflets from another valve. The Hancock II valve incorporates
second generation features such as low- pressure fixation, calcification
retardant treatment and a thinner stent. The MO II valve is
a modified Orifice valve with a modified scalloped sewing ring.
The Medtronic's intact valve is distinctive in that the calcification
retardant treatment colors it blue. It is fixed in zero pressure
leading the leaflets thinner and more flexible. Medtronic has
recently introduced the Mosaicm valve, which incorporates features
from both the intact valve and the Handcock II valve.
The Carpentier-Edwards standard porcine valve
became available shortly after the Hancock valve and has been
widely used. the frame of the valve is a flexible wire stent
intended to reduce stresses on the leaflets and orifice yet
retain its original contour over time. A one piece, cylindrical,
flexible Mylar support sur rounds a flexible wire frame. The
annulus is asymmetrical rather than circular to incorporate
the muscular septal ridge of the porcine right coronary cusp.
In the Carpentier-Edwards Supra Annular Valve, The mounting
structure of the aortic valve has been redesigned for the positioning
above rather than within the annulus.The fixation treatment
and the stent have been modified in an attempt to improve leaflet
durability. The sewing ring was reconfigured to increase the
effective orifice of the valve. The St. Jude BioImplant porcine
valve is available internationally. Clinical investigation has
begun on the X-Cell porcine valve, developed in conjunction
with St. Jude Medical and Hancock -Jaffe laboratories. The innovative
design features of this valve include an extraction process
to selectively remove calcification sites from the porcine tissue,
sterilization bygamma irradiation treatmento reduce leaflet
stiffness and a clothless stent. It also features zero-pressure
fixation and, in smaller (2.5mm) sizes, a composite leaflet
arrangement.
Porcine Heterograft Valves (Unstented)
The homograft is considered to have
properties superior to those of the heterograft with regard
to hemodynamics and thromboembolism. In an attempt to incorporate
some of the advantages of a homograft into an easily available
commercial product several manufacturers have recently begun
clinical testing of stentless porcine valves. This potential
benefit is achieved at the expense of a more difficult implant
technique. As with homografts there are potentially three ways
of implanting a stentless porcine valve :
(1)
as a replacement for the aortic root with reimplantation of
the coronary arteries;
(2)
as a miniroot replacement ,where the leaflets remain attached
to the donor aortic wall, which is inserted within the host
aorta; and
(3)
as a valve- only replacement,where the sides of the donor aorta
are scalloped and the valve is sewn freehand so free into the
the subcoronary position in the host's aorta.
Bovine Pericardial Heterograft Valves
Pericardial valves are assembled using biological
tissues as a fabric, rather than being harvested directly, as
are porcine valves. The theoretical advantage include more symmetrical
and complete opening for optimal hemodynamics, the opportunity
to allow extra tissue for eventual shrinkage, any higher intrinsic
percentage of collagen than in porcine valves. Since it is the
collagen that is cross- linked during fixation with glutaraldehyde,
a stronger and more durable tissue should result. The Ionescu-Shiley
was was the first commercially available pericardial valve,
but it had an unacceptable rate of structural failure and was
taken off the market. The Carpentier- Edward Pericardial Bioprosthesis
received FDA approval in 1991 and has become quite well accepted.
It uses a sophisticated method of mounting the leaflets to the
stent which does not depend on stitches passing through the
the leaflets (fig.107e-lower left).
The leaflets are secured behind the stent pillar by a plastic
plug, which serves as an anchor to prevent them from being pulled
through the opening in the wire frame. An international model
has a modified sewing ring, which is reinforced and more cone
-shaped.
Complications
(1) Structural
deterioration refers to any change in valve function resulting
from an intrinsic abnormality causing stenosis or regurgitation.
(2) Nonstructural
dysfunction: any abnormality resulting in stenosis or regurgitation
that is not intrinsic to the valve itself. This includes inappropriate
sizing, also called prosthesis- patient mismatch.
(3) Thromboembolism
includes any valve thrombosis or embolus except those secondary
to infection or hemorrhage. This includes any neurologic deficit
and any peripheral arterial emboli unless proved to have resulted
from another cause. Patients who do not awaken postoperatively
or who awaken with a stroke or myocardial infarction are excluded.
Valve thrombosis is listed as a subcategory of thromboembolism.
(4) Anticoagulant-related
hemorrhage includes any episode of internal or external bleeding
( in patients taking anticoagulants or antiplatelets) that is
fatal, causes a stroke, or serious enough to require hospitalization
or transfusion.
(5) The diagnosis
of prosthetic vave endocarditis is based on clinical criteria,
including an appropriate combination of positive blood cultures
and clinical signs or histologic confirmation at reoperation
or autopsy. Morbidity associated with active infection, such
as thromboembolism or paravalvular leak is included in this
category only.
The Comparative Clinical Performance
The choice between valve types involves a
tradeoff between an increased risk of thromboembolism-thrombosis-bleeding
complex for mechanical valves versus the structural deterioration
of tissue valves.
Structural Deterioration of Biological Valves
Structural valve deterioration with tissue
valves is not a constant risk event but increases with time.Thus
linearized rates are not appropriate and actuarial methods must
be used to describe and compare them.
Porcine Valves
Stented porcine prostheses represent by far
the most commonly used biological valves. Freedom from structural
deterioration at ten years ranges from about 60 to 90 percent
for aortic position and from about 60 to 80 percent from the
mitral position.
Pericardial Valves
After an initial unsatisfactory experience
to with the Ionescu-Shiley valve, which did not terribly but
was not equal to the standards of other contemporary bioprostheses,
the Carpentier-Edwards valve seems to have rescued the concept
of bovine pericardium as an acceptable alternative for valve
fabrication. There has now been over twelve years experience
with it, it has received FDA approval for marketing in the aortic
position, and the results to date have been encouraging.
Homografts
Thromboembolism rates for homograft valves
are considered quite low or even zero by some investigators.
For structural deterioration, homografts should be evaluated
according to the various methods of sterilization and preservation.
It appears that the series that used a chemical or irradiation
process for sterilization have the highest rates of failure,
about 40 percent structural valve deterioration free at ten
years. Those sterilize by antibiotic whether stored in nutrient
solution or cryopreserved,are more durable with about 75 percent
structural valve deterioration free at ten years.
Pulmonary Autograph
The pulmonary autograft used as an the aortic
valve replacement is considered a "permanent" valve and is especially
appropriate for young patients. Excellent results have also
been reported in treating endocarditisin. Freedom from replacement
for all reasons including endocarditis has been reported to
be as high as 85 percent at twenty years but has also been found
to be 48.5% at 19 years.
Choice of Valve
The embolic-thrombosis-bleeding complex with
mechanical valves and structural failure with biological valves
serve to distinguish between the two valve types. But, judging
from the wide variation in reported results with each model
of valve, patient-specific factors must influence the results
more than valve-specific ones, and it is impossible to rank
valves, within valve types, on the basis of complication rates.
However, some general recommendations can be made with regard
to valve selection. Though not covered in this review, valve
repair, when practical, should be considered preferable to replacement,
especially in the mitral position, but also the aorctic positions.
When replacement is necessary an argument can be made for a
particular class of valve under certain circumstances. A biological
valve should be used when the patient cannot or will not take
anticoagulants, desires pregnancy, or has a short life expectancy.
A mechanical valve should be used if the patient will be on
anticoagulants anyway (because of a trial fibrillation or mechanical
valve in another position), is in renal failure or on dialysis,
or has a long life expectancy. Mechanical valves should also
be considered first for double valve replacement, because the
thromboembolic risk is not an additive with two valves but the
risk of structural deterioration is additive.
Future Developments
The current trend in mechanical valves is
toward further development of bileaflet valve principle enhancing
the very successful St. Jude valve design. New directions include
the search for better hemodynamics, for example parallel leaflets
and lower thrombogenicity Better anticoagulant management has
the potential to reduce bleeding complications and also to reduce
thromboembolic events. If markers of thrombogenicity can be
identified and measured preoperatively, tissue valves can be
preferentially used in high risk patients and mechanical valves
and low risk patients, even an older ages. The primary advantage
of biological valves is a reduced need for anticoagulation,
but the offsetting disadvantage is poorer durability. The search
for im prve durability will define the newer generation of biological
valves. A major factoris low or zero pressure fixation, which
allows the leaflets to be fixed in the neutral position and
to retain more of the natural flexibility. Anticalcification
treatments may improve durability and the elimination of the
stent should provide improved with hemodynamics.
Reference:Grunkemeier,G.L. and others,Hurst's
The Heart Update I,1996,Pp.98-123.