Type
1. Sustained Ventricular Tachycardia
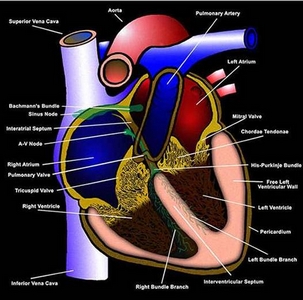
It may arise in the conducting system below
the bundle of His or in the ventricular myocardium or both (figures
104b,
104c),
lasting 30s or more at rate of 100 beats/min.or more. It is
generally life threatening unless there is no structural heart
disease (see: fig.7,
fig.8,
fig.9a,
fig.9b,
fig.10).
With prior myocardial infarction (MI, heart
attack) and ventricular aneurysm (an abnormal dilatation of
a portion of the myocardium caused by a thinning of the heart
muscle due to a MI, (see fig.10,
fig.51a)
a sustained monomorphic VT may be hemodynamically well tolerated.
This type can be treated with intravenous (IV) drugs.
But with transient myocardial ischemia, the
VT may be polymorphic or sinusoidal, and hemodynamically unstable
with a higher risk of VF than the momomorphic type. With unstable
blood pressure (BP) in acute myocardial infarction, immediate
electrical cardioversion is indicated (fig.
11, fig.12).
Proarrhythmia due to prior drugs for VT should
be suspected if the VT morphology is different from a prior
VT form, especially if prior drugs or changes have been prescribed,
with a prolonged QT (see figure
94) interval, or if the new VT has a polymorphic or torsades
de pointe configuration (see fig.13).
If there are repeated recurrences after
cardioversion, the possibility of proarrhythmia should be entertained
and temporary pacing ( fig.84b, fig.85,
fig.86,
fig.87,
fig.88,
fig.89,
fig.90,
fig.91,
fig.92
) may be useful. Other causes of repeated recurrence include
ischemia, heart failure, autonomic surges, or electrolyte disturbances.
Acute Management of Sustained Monomorphic VT
This form of V.T. (see figure
9b) may occur in acute or chronic ischemic heart disease,
idiopathic dilated, or hypertrophic cardiomyopathy (see fig.61,
fig.62)
and less frequently in inflammatory or infiltration diseases,
or a primary electrical disturbance .
In acute myocardial infarction, sustained
VT occurs most commonly within 24 hours of the onset. It carries
a risk of degenerating into ventricular fibrillation (VF ) (see
figure
9b ) and must be treated aggressively. A lidocaine (heart
medication) intravenous ( IV) bolus followed by a continuous
infusion may be tried. If the VT does not revert immediately
or if the patient is hypotensive (low blood pressure) immediate
DC electrical cardioversion is required. Following the cardioversion,
the IV infusion of lidocane is continued to prevent recurrences.
If the VT recurs despite lidocane, boluses
of procainamide (another heart drug) are infused. If breakthroughs
continue, other heart drugs like bretylium tosylate or intravenous
amiodarone are indicated. The therapy may be stopped after 48-72
hours, since the risk of recurrence is small at that point.
| Type2:
Sustained VT in convalescent phase of acute myocardial infarction |
A second category of sustained VT (see fig.9b,
fig.10,
fig.51a
) related to acute myocardial infarction occurs in the convalescent
period, and is most common in patients with large anterior left
ventricular wall infarctions. Management is similar to the above
therapy. This type has a high death rate.
Long Term Management of VT in Chronic Ischemic
Heart Disease
Approaches include the following:
1) Antiarrhythmic therapy guided
by invasive electrophysiologic testing, exercise EKG testing,
or ambulatory EKG monitoring,
2) Surgical procedures to excise
or cryoablate reentrant pathways or automatic foci,
3) catheter ablation (see fig.11),
4) ICD (implantable cardioverter
defibrillator) (see fig.12,
fig.61,
fig.62)
A) Repetitive
Monomorphic VT
This
is an uncommon form of repetitive, nonsustained VT, but it may
become sustained. It is more common in women and is usually
benign. The QRS in the EKG suggest a left bundle branch block
(LBBB) pattern with a widened QRS (see fig.8).
Treatment is indicated when there is structural disease or severe
symptoms. Beta adrenoceptors and calcium (Ca 2+) entry blocking
agents are effective in some patients. Catheter ablation is
an option if symptomatic.
B) Nonsustained Ventricular Tachycardia (V.T.)
It represents nonsustained runs of VT (salvos
of 3 to 5 consecutive impulses or nonsustained VT of 6 impulses
to 30s) (see fig.7)
which are considered indicators of high risk for potentially
fatal arrhythmias (sustained VT or ventricular fibrillation,
VF) in most clinical settings. But patients with no organic
disease may not be at increased risk.
Even without increased risk, severe symptoms
like transient syncope or near syncope require therapy. Some
of the highest risk patients have cardiomyopathy (see fig.40b,
fig.40c,
fig.40d,
fig.40f,
fig.41,
fig.43a,
fig.43b)
and advanced coronary heart disease (fig.51a,
fig.53,
fig.54,
fig.55,
fig.56a).
Implantable cardioverder defibrillator (ICD) therapy (see fig.11,
fig.12)
has shown a greater decreased mortality than the best conventional
therapy.
LESS
COMMON FORMS OF VT
1)
Brugada syndrome is an inherited genetic disorder affecting
the repolarization function of the sodium channel associated
with the presence of an RSR' in lead V1 with coving of the ST
segment in leads VI-V3 and a normal QT interval. This syndrome
is clinically characterized by ventricular tachyarrhythmias
and sudden death. The symptomatic patient requires treatment
with an implantable cardioverter defibrillator (ICD). A slightly
different abnormality of the same gene is responsible for one
of the long QTsyndromes (LQT3).
2)
The "long QT syndrome" (LQTS) is associated with a
prolonged QT interval during sinus rhythm signifying an inherited
or acquired repolarization disorder. The inherited form of LQTS
has as many as six different responsible genotypes with several
phenotypes, and can predispose to a special kind of VT called
"torsade de pointes". This VT has a characteristic
polymorphic EKG appearance of the QRS, which appears to be"
twisting about on its points" (see fig.13).
A fairly specific electrophysiologic mechanism, "early
afterdepolarizations", caused by a malfunctioning sodium
or potassium channel, appears to be responsible for torsade
de pointes, which can be treated by drugs or an ICD. Some youngsters
present with seizures due to hypotension from the torsade de
pointes. Some patients predisposed to drug-induced, acquired
LQTS may actually have an inherited form that makes them vulnerable
to the QT prolonging, proarrhythmic effects of many drugs.
3) Several types
of cardiomyopathies can cause ventricular tachyarrhythmias and
may show uniquely appearing EKGs during sinus rhythm. As an
example, arrhymogenic right ventricular cardiomyopathy can be
characterized during sinus rhythm by a epsilon wave (a notch
on the terminal portion of the QRS in lead V1 due to delayed
right ventricular activation) and T wave negativity in the anterior
precordial leads.This disorder, due to fatty replacement of
right and, less commonly, left ventricular myocardium, can result
in lethal ventricular arrhythmias. Patients with hypertrophic
cardiomyopahy (see fig.39a,
fig.39b,
fig.40a,
fig.40b)
can exhibit large voltages consistent with ventricular hypertrophy
and deep Q waves often confused with myocardial infarction,
whereas patients who have muscular dystrophy can have tall R
waves in the anterior precordial leads suggestive of a true
posterior myocardial infarction. Patients with dilated cardiomyopathy
can have VT due to bundle branch reentry that has a LBBB contour
and can be treated with radiofrequency catheter ablation.
4) Two types of
VTs that occur in patients with apparently structurally normal
hearts include those coming from the right ventricular outflow
tract that have a LBBB-inferior axis morphology, and those coming
from the left ventricular septum that have a RBBB and left axis
deviation contour. Both are relatively easily eliminated with
radiofrequency ablation.
Reference:Zipes.D.P.,Clinical Application of
the Eleectrocardiogram,JACC Vol.36,No.6,2000:1746-8
TREATMENT OF CARDIAC ARRHYTHMIAS WITH DILANTIN
CARDIAC ARRHYTHMIAS
The first use of PHT(Phenytoin) in cardiac
disorders was reported by Leonard in 1958. Since it was the
pioneer paper in this field, it will be summarized in some detail.
It brings into focus three important points which develop throughout
the literature:
1) PHT is an effective antiarrhythmic, prompt
in its action;
2) PHT has a high margin of safety;
3) In the
acute stage, substantial amounts of PHT may be required, adjusted
to the severity of the condition.
Leonard, Archives of Internal Medicine (1958), demonstrated
the beneficial effect of PHT in controlling ventricular hyperirritability
complicating myocardial infarction in a patient. The patient
was gravely ill with cardiographic findings of typical ventricular
tachycardia. In spite of the previous history of complete heart
block, it was felt that intravenous procainamide, if carefully
controlled, was the treatment of choice. The patient was receiving
Arterenol to maintain his blood pressure at 110/70. Procainamide
was given intravenously. During a period of approximately two
hours, 2300 mg of procainamide was given, in spite of several
episodes of marked hypotension, but finally discontinued because
of disturbing widening of the QRS complex without reversion
to a normal sinus mechanism. The patient's condition remained
critical, and it was considered advisable to investigate the
therapeutic potential of intravenous PHT. PHT was administered
slowly intravenously in a dose of 250 mg. A cardiogram recorded
approximately two minutes later revealed a normal sinus mechanism
coupled with premature auricular contractions. In twenty minutes
ventricular tachycardia had recurred. An immediate additional
dose of 250 mg of PHT was given and within moments a normal
sinus mechanism appeared. Four hours later ventricular tachycardia
returned and was again successfully reverted to a normal sinus
rhythm with 250 mg of intravenous PHT. Because the duration
of effectiveness of PHT was unknown, a constant, slow intravenous
infusion of 250 mg of PHT was started. The normal sinus mechanism
was maintained in this fashion for successive periods of six
and four hours. At these intervals ventricular tachycardia returned,
but was promptly reverted with additional intravenous doses
of 250 mg of PHT. At this time it was considered advisable to
supplement the intravenous therapy with 3 grains of PHT and
500 mg of procainamide every four hours orally. Eighteen hours
after its initiation the intravenous PHT was discontinued. An
electrocardiogram at this time showed posterior myocardial infarction
with a normal sinus mechanism. On the following day procainamide
was discontinued, and the patient was maintained with 3 grains
of PFU orally every six hours. There was no recurrence of signs
of ventricular irritability. The patient made an uneventful
recovery. The author suggests that PHT may represent a drug
with a wide margin of safety that is effective in controlling
serious ventricular hyperirritability.
Leonard, W. A., Jr., The use of diphenylhydantoin
(Dilantin) sodium in the treatment of ventricular tachycardia,
A.M.A. Arch. Intern. Med., 101: 714-717,1958. Paramedic Medications,
DrugCard, Revised 5/01
DILANTIN (PHENYTOIN)
CLASS:
Anticonvulsant
ACTION:
1. Decreases Neuron Excitability thru Increasing
Sodium efflux from neurons.
2.Shortens QT interval,decreasing serum levels
of quinidine, dilitiazem, disopypramide
INDICATIONS:
1. Status Epilepticus
2. Digitalis induced dysrhythmias
3.Torsade de Pointes
CONTRAINDICATIONS:
1. Hypersensitivity
2. Bradycardia
3. High Degree HB's (2 & 3)
PRECAUTIONS:
1. Do not administer faster that 50 mg/min..
Causes Hypotension.
2. DO NOT mix in D5W. PRECIPITATION OCCURS
SIDE EFFECTS:
1. Hypotension
2. Heart block
3. Dysrhythmias
4. Respiratory Depression
5. CNS Depression
6. Nausea, vomiting, blurry vision
DOSAGE:
Seizures: 10-20 mg/kg SLOW IVP. Do
not exceed 1 gm or rate of 50 mg/min.
Dysrhythmias: 50-100 mg SLOW IV every 5-15
min as needed to max of 1 gm.
ROUTE: IV
HOW SUPPLIED
Ampule 250 mg/5 mi
PEDIATRIC DOSAGE.
Seizures: 10-20 mg/kg SLOW IVP. (1-3
mg/kg/min) Same Precautions as adult.
Dysrhythmias: 5 mg/kg SLOW IV. Same precautions
as adult.
TORSADE DE POINTES
e.mail:www.emedicine.com/EMERG/topic596.htm
Last Updated: May 30. 2002
Author: Michael Bessette, MD, Associate Director, Assistant
Professor, Department of Emergency Medicine. Mount Sinai School
of Medicine
Coauthor(s): Sheldon Jacobson. MD, Chair, Professor, Department
of Emergency Medicine, Mount Sinai Medical Center
Background:
Torsade de pointes (TDP), often referred to
as torsade, is an uncommon variant of ventricular tachycardia
(VT). The underlying etiology and management of torsade are,
in general, quite different from those of garden-variety VT.
The management of torsade with group IA antidysrhythmic drugs
can have disastrous consequences. Differentiating between these
entities, therefore, is critically important.
Pathophysiology:
Torsade is defined as a polymorphous VT in
which the morphology of the QRS complexes varies from beat to
beat. The ventricular rate can range from 150 beats per minute
(bpm) to 250 bpm. The original report described regular variation
of the morphology of the QRS vector from positive to net negative
and back again. This was symbolically termed torsade de pointes,
or "twisting of the point" about the isoelectric axis,
because it reminded the original authors of the torsade de pointes
movement in ballet. Most cases exhibit polymorphism, but the
axis changes may not have regularity.
The definition also requires that the QT interval
be increased markedly (usually to 600 msec or greater).
Cases of polymorphous VT, which are not associated
with a prolonged QT interval, are treated as generic VT.
Torsade usually occurs in bursts that are
not sustained; thus, the rhythm strip usually shows the patient's
baseline QT prolongation.
The underlying basis for rhythm disturbance
is delay in phase III of the action potential. This prolonged
period of repolarization and the inhomogeneity of repolarization
times among myocardial fibers allow the dysrhythmia to emerge.
The initiating electrophysiologic mechanism may be triggered
activity or reentry.
Six genetic variants currently are recognized.
Genotypes LQT1 and LQT2 have slow potassium channels, while
LQT3 shows defects in the sodium channels. Treatment modalities
soon may be based on the genotype of the individual.
Frequency:
In the US: Incidence of torsade is still
unknown.
Mortality/Morbidity:
In the US, 300,000 sudden cardiac deaths occur
per year. TDP probably accounts for fewer than 5%.
Sex:
Women are 2-3 times more likely to develop
TDP than men.
Women have more QT prolongation secondary to drug therapy.
Congenital long QT syndrome is autosomal dominant but shows
greater frequency of expression and a greater lengthening of
the QT interval in women than in men.
Age:
The highest frequency is in patients
aged-35-50 years.
History:
Ask patient about previous cardiac events
or syncope and any medications that the patient presently is
using.
History of congenital deafness or family history of sudden death
may indicate a long QT syndrome.
Physical:
No physical findings are typical of TDP.
Causes:
Prolongation of the QT interval may be congenital, as seen in
the Jervell and Lange-Nielson syndrome (ie, congenitally long
QT associated with congenital deafness) and the Romano Ward
syndrome (ie, isolated prolongation of QT interval). Both of
these syndromes are associated with sudden death due to either
primary ventricular fibrillation or torsade that degenerates
into ventricular fibrillation.
Prolonged QT is found in only 0.25-0.3% of
deaf-mute children.
The acquired conditions that predispose one
to torsade either decrease the outward potassium current or
interfere with the inward sodium and calcium currents, or fluxes.
The electrolyte disturbances that have been
reported to precipitate torsade include hypokalemia and hypomagnesemia.
Hypokalemia and hypomagnesemia, in turn, cause
a delay in phase III (ie, reprolongation) and form the substrate
for emergence of the dysrhythmia.
Antiarrhythmic drugs reported to be etiologic
include class IA agents (eg, quinidine, procainamide, disopyramide),
class IC agents (eg, encainide, flecainide), and class III agents
(eg, sotalol, amiodarone).
Drug interactions with the antihistamines,
astemizole (recalled from US market), and terfenadine (recalled
from US market) can precipitate torsade; these drugs should
never be used with class IA, IC, or III agents.
Astemizole and terfenadine, in high dosages
or when used in combination with the azole antifungal drugs
or the macrolide antibiotics, have been reported to precipitate
torsade and sudden death,
Grapefruit juice has been shown to slow the
hepatic metabolism of these antihistamines as well as other
drugs and to prolong the QT interval in patients taking astemizole
or terfenadine (recently taken off the market by the US Food
and Drug Administration [FDA]).
Clinical implications of this interaction
are unclear.
Other drugs that prolong the QT interval
and have been implicated in cases of torsade include phenothiazines,
tricyclic antidepressants, lithium carbonate, and anthracydine
chemotherapeutic agents (eg, doxorubicin, daunomycin).
Risk factors:
Female gender
History of syncope or resuscitated arrest
Family history of sudden death
Lab Studies:
Potassium, magnesium, and calcium levels
Other tests:
ECG: Once in sinus rhythm, examine the QTc
interval. See Picture 1 and Picture 2 below for typical examples,
as well as figures fig.11, fig.12,
fig.13, fig.62
and fig.94.
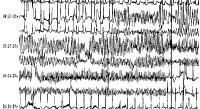
Picture 1 (rhythm strip including animation). A run of torsade
de pointes in a 70-year-old man who developed QT prolongation
(QTc = 0.61 sec) secondary to quinidine therapy. The bottom
strip shows resolution with overdrive ventricular pacing (see
also figures: fig.11, fig.12,
fig.13, fig.62
).
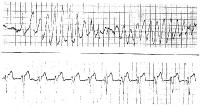
Picture 2 (rhythm strip including animation). A patient with
prolonged QT and atrial ectopy. Strip shows premature beat that
entrains and a run of torsade de pointes (see figures fig.11,
fig.12, fig.13,
fig.62 ).
Prehospital Care:
Institute immediate advanced cardiac
life support (ACLS) protocol for VT,
Overdrive pacing may be necessary at a rate of up to 140 bpm
to control the rhythm (see picture 1 above).
Emergency Department Care:
Torsade, an inherently unstable rhythm,
is prone to revert to more stable rhythms spontaneously and
prone to recurrences. Torsade also is subject to degeneration
into ventricular fibrillation. Begin therapy as soon as the
rhythm clearly fulfills the criteria for torsade.
Treat hypokalemia if it is the precipitating
factor and administer magnesium sulfate in a dose of 2-4 g intravenously
(IV) initially.
Magnesium usually is very effective even in
the patient with a normal magnesium level.
If this fails, repeat the initial dose, but danger of hypermagnesemia
(depression of neuromuscular function) requires close monitoring.
Other therapies include overdrive pacing and isoproterenol infusion.
Most (75-82%) TDP rhythms are started by a pause. Pacing at
rates up to 140 bpm may prevent the ventricular pauses that
allow TDP to originate.
The patient with torsade who is in extremis should be treated
with electrical cardioversion or defibrillation.
Anecdotal reports cite successful conversion
with phenytoin (Dilantin) and lidocaine.
Patients with congenital long QT syndromes are thought to have
an abnormality of sympathetic balance or tone and are treated
with beta-blockers. If the patient breaks through this therapy
and enters the ED in torsade, a short-acting beta-blocker, such
as esmolol, can be tried.
A few cases of successful conversion using phenytoin and overdrive
pacing have been reported.
If patient is unresponsive to conversion with phenytoin and
overdrive pacing, attempt electrical cardioversion.
Cervical sympathectomy and implantable pacemaker/defibrillator
have been used in some cases for long-term management.
Shortening the action potential decreases the likelihood of
immediate recurrence.
Pacing or administration of isoproterenol
to a rate of 90-100 bpm is effective.
Withdraw all QT-prolonging drugs.
Consultations:
Immediate cardiology evaluation and
follow-up are required.
Magnesium and potassium are first-line therapies
in the treatment of torsade de pointes. Isoproterenol and short-acting
beta-blockers also have been used. For treatment of primary
torsades associated with congenital prolonged QT syndromes,
use a beta-blocker. In primary and secondary torsade, overdrive
pacing is an appropriate secondary therapy. In treatment of
recurrent torsade, implantable defibrillators are used as prophylaxis.
If the patient is hemodynamically unstable, carry out electrical
cardioversion or defibrillation at once.
Drug Category:
Electrolytes :
These agents are therapeutic alternatives for the treatment
of torsade de pointes. Assessment of patient for underlying
electrolyte abnormalities that may cause refractory dysrhythmia
is important. Some of the electrolyte abnormalities associated
with torsade de pointes include hypokalemia and hypomagnesemia.
Electrolytes also reduce the arrhythmic effects of offending
drugs.
Drug Name
Magnesium sulfate -
DOC for treatment of torsade de pointes. Acts as antiarrhythmic
agent and diminishes frequency of PVCs, particularly when secondary
to acute ischemia. Deficiency in this electrolyte is associated
with sudden cardiac death and can precipitate refractory VF.
Magnesium supplementation is used for treatment of torsade de
pointes, known or suspected hypomagnesemia, or severe refractory
VF.
Adult Dose
1-2 g IV diluted in 100 mL of D5W over 1-2 min; may repeat
q4h with close monitoring of deep tendon reflexes
Pediatric Dose
Torsade de pointes: Not established
Hypomagnesemia: 25-50 mg/kg/dose q4-6h for 3-4 doses; single
dose not to exceed 2 g also may be administered and repeated
if hypomagnesemia persists
Contraindications
Documented hypersensitivity; heart block;
Addison disease, myocardial damage; severe hepatitis
Interactions
Nifedipine may cause hypotension and neuromuscular
blockade; may increase neuromuscular blockade seen with aminoglycosides
and potentiate neuromuscular blockade produced by tubocuranne,
vecuronium, and succinylcholine; may increase CNS effects and
toxicity of CNS depressants and betamethasone and cardiotoxicity
of ritodrine
Pregnancy
A - Safe in pregnancy
Precautions
Magnesium may alter cardiac conduction, leading
to heart block in digitalized patients; respiratory rate, deep
tendon reflexes, and renal function should be monitored when
administered parenterally;
caution when administering since may produce significant hypertension
or asystole; in overdose, calcium gluconate, 10-20 mL IV of
10% solution, can be given as antidote for clinically significant
hypermagnesemia
Drug Name
Potassium chloride (Klor-Con, K-Dur, Micro-K) -- First-line
therapy in treatment of torsade de pointes. Essential for maintenance
of intracellular tonicity, transmission of nerve impulses, contraction
of cardiac, skeletal, and smooth muscles, and maintenance of
normal renal function. Gradual potassium depletion occurs via
renal
excretion, through GI loss, or because of low intake. Depletion
usually results from diuretic therapy, primary or secondary
hyperaldosteronism, diabetic ketoacidosis, severe diarrhea (if
associated with vomiting), or inadequate replacement during
prolonged parenteral nutrition. Depletion sufficient to cause
1 mEq/L drop in serum potassium requires a loss of about 100-200
mEq of potassium from the total body store.
Adult Dose
Serum levels >2.5 mEq/L: 10 mEq over
1 h prn based on frequently
obtained lab values; not to exceed 200 mEq/d
Serum levels <2.5 mEq/L: 40 mEq over 1 h prn based on frequently
obtained lab values; not to exceed 400 mEq/d
Pediatric Dose
1 mEq/kg IV over 1-2 h prn based on frequently
obtained lab values
Medical/Legal Pitfalls:
Failure to recognize as separable from
other forms of VT
If one fails to differentiate this rhythm
disturbance, the therapy of the dysrhythmia is likely to include
a type IA antidysrhythmic agent, such as procainamide.
Type IA agents perpetuate the rhythm disturbance
in torsade.
Contraindications
Hyperkalemia; renal failure; conditions in
which potassium is retained; oliguria; azotemia; crush syndrome;
severe hemolytic reactions; anuria; adrenocortical insufficiency
Interactions
ACE inhibitors may result in elevated serum
potassium concentrations; potassium-sparing diuretics and potassium-containing
salt substitutes can produce severe hyperkalemia; in patients
taking digoxin, hypokalemia may result in digoxin toxicity;
use caution if discontinuing a potassium preparation in patients
maintained on digoxin
Pregnancy
A - Safe in pregnancy
Precautions
Do not infuse rapidly; high plasma concentrations of potassium
may cause death due to cardiac depression, arrhythmias, or arrest;
plasma levels do not necessarily reflect tissue levels; monitor
potassium replacement therapy whenever possible by continuous
or serial ECG; when concentration >40 mEq/L infused, local
pain and phlebitis may follow.
Drug Category:
Adrenergic agonist
These agents alter the electrophysiologic mechanisms responsible
for arrhythmic disturbances.
Drug Name
Isoproterenol (Isuprel)
Stimulates beta l- and beta 2-adrenergic receptor activity.
Binds beta-receptors of heart, smooth muscle of bronchi, skeletal
muscle, skeletal vasculature, and alimentary tract. Positive
inotropic and chronotropic actions.
Adult Dose
1 mL of 1:5000 solution (0.2 mg) diluted in 10 mL sodium chloride
or 5% dextrose injection
0.02-0.06 mg IV (1-3 mL of diluted solution) initially
0.01-0.2 mg IV (0.5-10 mL of diluted solution) subsequent doses
to
achieve heart rate of 90-100 bpm
Alternatively, 10 mL of 1:5000 solution (2 mg) diluted in 500
mL of D5W, or 5 mL of 1:5000 solution (1 mg) diluted in 250
mL of D5W 5 mcg/min (1.25 ml/min of diluted solution) subsequent
doses to achieve heart rate of 90-100 bpm
Pediatric Dose
Not established
AHA recommends initial infusion rate of 0.1 mcg/kg/min; titrate
to HR effect
Contraindications
Documented hypersensitivity; tachyarrhythmias; tachycardia
or heart block caused by digitalis intoxication; ventricular
arrhythmias that require inotropic therapy; angina pectoris
Interactions
Bretylium increases action of vasopressors on adrenergic receptors,
which may in turn result in arrhythmias; guanethidine may increase
effect of direct-acting vasopressors, possibly resulting in
severe hypertension; tricyclic antidepressants may potentiate
pressor response of direct-acting vasopressors.
Pregnancy
C - Safety for use during pregnancy has not been established.
Precautions
By increasing myocardial oxygen requirements while decreasing
effective coronary perfusion, isoproterenol may have deleterious
effect on injured or failing heart; isoproterenol may worsen
heart blocks or precipitate Adams-Stokes attacks in some patients,
presumably with organic disease of AV node and its branches;
caution with coronary artery disease, coronary insufficiency,diabetes,
hyperthyroidism, patients sensitive to sympathomimetic amines;
if HR exceeds 110 bpm, may be advisable to decrease infusion
rate or temporarily discontinue infusion
Drug Category:
Beta-adrenergic blocker
This agent is excellent for use in patients at risk for experiencing
complications from beta-blockade, particularly with reactive
airway disease, mild to moderately severe left ventricular dysfunction.
and peripheral vascular disease. The short half-life of 8 min
allows for titration to desired effect and ability to stop quickly
if needed.
Drug Name
Esmolol (Brevibloc)
Ideal for use in patients at risk for experiencing complications
from beta-blockade, especially patients diagnosed with mild
to moderately severe LV dysfunction and those with peripheral
vascular disease. Has short half-life of 8 min; thus, easily
titratable to desired effect. Therapy may be stopped quickly
prn.
Adult Dose
Initially, 500 mcg/kg/min IV infusion for 1 min followed by
4-min maintenance infusion of 50 mcg/kg/min; if adequate therapeutic
effect not observed within 5 min, repeat loading dose and follow
with maintenance infusion of 100 mcg/kg/min; continue titration
procedure, repeating loading infusion and increasing maintenance
infusion by increments of 50 mcg/kg/min for 4 min
Pediatric Dose
Not established; suggested dose 100-500 mcg/kg administered
over 1 min
Contraindications
Documented hypersensitivity: uncompensated congestive heart
failure; bradycardia; cardiogenic shock; AV conduction abnormalities
Interactions
Aluminum salts, barbiturates, NSAIDs, penicillins, calcium
salts, cholestyramine, and rifampin may decrease bioavailability
and plasma levels, possibly resulting in decreased pharmacologic
effect; sparfloxacin, astemizole, calcium channel blockers,
quinidine, flecainide, and contraceptives may increase cardiotoxicity;
digoxin, flecainide, acetaminophen, clonidine, epinephrine,
nifedipine, prazosin, haloperidol, phenothiazines, and catecholamine-depleting
agents increase toxicity
Pregnancy
C - Safety for use during pregnancy has not been established.
Precautions
Beta-adrenergic blockers may mask signs and symptoms of acute
hypoglycemia and clinical signs of hyperthyroidism; symptoms
of
hyperthyroidism, including thyroid storm, may worsen when medication
withdrawn abruptly; withdraw drug slowly and monitor patient
closely
Further Inpatient Care:
Admit patient to ICU for continued monitoring and withdrawal
of offending drugs.
Transfer:
Since ICU care is warranted, transfer patient to a facility
with acute cardiac care capabilities.
Deterrence/Prevention:
Avoid QT-prolonging medications.
Patients with TDP have a 50% chance of a recurrence even with
therapy.
Medical/Legal Pitfalls:
Failure to recognize as separable from other forms of VT
If one fails to differentiate this rhythm disturbance, the
therapy of the dysrhythmia is likely to include a type IA antidysrhythmic
agent, such as procainamide.
Type IA agents perpetuate the rhythm disturbance in torsade
de pointes.